Россия
Россия
Россия
Рассматривается комплексная оценка рыбоводно-биологических и физиолого-биохимических показателей стерляди при использовании в кормлении биологически активных компонентов из сырья пустырника сердечного, или пустырника обыкновенного (Leonurus cardiaca L., 1753), и зизифоры тонкой (Ziziphora tenuior L., 1753). В качестве объектов использовали особей стерляди (Acipenser ruthenus Linnaeus, 1758). После 10 суток адаптации выращиваемые объекты переводили на комбикорма (эксперимент длился 30 суток). Особи были разделены на контрольную и две опытные группы, предполагавшие дополнительно к диете 1 Leonurus cardiaca – 1 % и к диете 2 Ziziphora tenuior – 1 %. Исследования статистической связи значений измеряемых показателей состояния сердечно-сосудистой системы и общего состояния организма установили положительное влияние на физиолого-биохимический статус рыб при включении добавок. Повысилась концентрация общего белка на 16–42 %, гемоглобин увеличился на 14–44 %. Анализ кардиосоматического индекса выявил его снижение относительно контроля для второй опытной группы на 8,05 %, что подтвердило повышение кардиопротекторной функции добавки и адаптивный эффект к действию дефицитбелковой диеты, обусловленный низким качеством комбикормов. В опытных группах зафиксировано повышение активности АСТ и АЛТ по сравнению с контролем, что привело к значительному увеличению коэффициента де Ритиса – на 39–85 %, что свидетельствует об усилении белковосинтезирующей функции печени и улучшении защитно-приспособительных механизмов организма в целом, что выразилось в более полном усвоении питательных веществ корма и росте массы на 5–16 % при снижении конверсии комбикорма на 7,6–18,2 % относительно контроля. Присутствие на кормовом рынке фальсифицированных кормовых компонентов выявило необходимость применения терапии для снижения рисков проявления последствий чрезмерной кардионагрузки. В поиске решений данной проблемы стала актуальной разработка комбикормов, стабилизирующих физиологический статус в случае возникновения рисков нарушений в работе сердечно-сосудистой системы, которые могут происходить вследствие влияния неблагоприятных факторов при интенсивном выращивании. Возможным вариантом решения такой проблемы может стать применение в составе кормов кардиопротекторов, которые улучшат хозяйственно-важные признаки исследуемого вида рыб и снизят вероятность ухудшения состояния и гибели в процессе выращивания за счет уменьшения риска возникновения сердечно-сосудистых заболеваний. Полученные в ходе выполнения работы оценки изменений функционального состояния сердца ценных видов рыб позволили установить оптимальные дозы введения натуральных кардиопротекторов Leonurus cardiaca и Ziziphora tenuior в состав рецептур соответствующих комбикормов для стерляди в количестве 1 %.
фитобиоактивные растительные ингредиенты, кардиопротекторные свойства, комбикорм, кардиосоматический индекс
Introduction
Over the past decade, there have been significant losses in fish farms due to diseases affecting the cardiovascular system of fish. Cases of high fish mortality linked to cardiac abnormalities have been reported in salmon commercial farms [1, 2].
In a stressful state, not only endurance increases, but also changes occur at all levels of functional activity of fish organ systems, the cardiovascular system is no exception; under the influence of adverse factors, both quantitative and qualitative indicators of it change [3]. Physiological and morphological disorders of the heart in fish were often observed: an increase in heart mass (cardiosomatic index), scars on the heart, an uneven edge of the heart, an expansion of the heart chamber, and hemorrhagic inclusions on the heart. It was found out that with intensive cultivation and increased growth rate, the heart of fish is exposed to high biological and stress loads, which can damage the vascular endothelium of the heart [4-6].
Such injuries reduce the fish's ability to meet the metabolic demands of physical activity, higher temperatures, and low oxygen levels [7]. Additionally, cultivated objects at sturgeon hatcheries experienced an increase in the cardiosomatic index, directly affecting the efficiency of the sturgeon cultivation process [8]. It is also believed that fish experience heart failure due to stressful situations that can arise at any stage of production. These stressful situations can be caused by various factors such as sudden changes in water temperature, transportation, handling, sorting, and treatment for diseases and parasites [9-11].
When these factors affect the fish, they can lead to problems with the cardiovascular system. This means that the fish may be at a higher risk of having a heart attack, developing ischemic disease due to a lack of oxygen (hypoxia), or experiencing symptoms of tachycardia. To find solutions for these problems, it has become important to develop compound feeds that can stabilize the physiological state in case of disorders in the cardiovascular system caused by adverse factors during intensive cultivation.
This research aims to tackle a problem encountered in the cultivation of sturgeon fish. The issue lies in a factor that significantly hampers the efficiency of the technological process for growing sturgeon. This factor is an increase in the cardiosomatic index during the growth of individuals. This increase indicates a deterioration of the cardiovascular system and an elevated risk of heart attack, particularly when exposed to various stressors. Currently, the main diagnostic methods used at enterprises are focused on detecting inflammatory processes (by measuring the total protein content in the blood) and conducting biochemical analysis of meat products to assess their quality. However, there are no diagnostic tools available for identifying diseases of the cardiovascular system, despite the fact that such conditions are quite common and widespread.
The global scientific community has become increasingly interested in a range of plant materials that are rich in essential oils and bioflavonoids. These substances are widely used in herbal medicine, pharmaceuticals, cosmetics, food products, and medical practice. The effects of excessive cardio loading can be mitigated by adjusting the composition of the feed used. One possible solution to this issue is the use of new ingredients with cardioprotective properties. These ingredients can enhance the economically important characteristics of the fish species under study and reduce the risk of deterioration and death during cultivation. This is achieved by minimizing the likelihood of cardiovascular diseases in fish farms caused by stress factors associated with the farming process. Therefore, conducting a patent search and analyzing international scientific literature, we selected the names of raw materials and plant-based preparations to compile a list of potential safe ingredients of local origin for cardioprotectors. We then evaluated the potential of using these ingredients as components in compound feeds for fish, considering the nutritional needs of sturgeon. This process allowed us to identify some promising ingredients for experimental formulations. These include Leonurus cardiaca and Ziziphora tenuior. Leonurus cardiaca (motherwort) is a perennial herbaceous plant of the family Lamiaceae, most in demand as a medicinal product in cardiology, the raw materials of which include polysaccharides (6.2%), organic acids (2.7%), tannins (2.35%), alkaloids (0.3%), ascorbic acid (0.27%), carotenoids (0.24%), flavonoids (0.23%), macronutrients: calcium, magnesium, iron; trace elements: copper, zinc, manganese [12]. Motherwort alkaloid compounds contribute to the correction of metabolic disorders caused by stress and anxiety, can restore normal heart rhythm and blood pressure. In addition, motherwort grass contains minerals important for the cardiovascular system – magnesium, sodium and potassium, as well as glycosides and organic acids, which have cardiotrophic and angioprotective properties. In addition to stress-protective properties, biologically active substances - flavonoids protect the body from the negative effects of free radicals, maintain normal vascular wall tone, improve blood clotting and capillary circulation, and these properties of motherwort are manifested only under conditions of oxidative stress, when the body requires antioxidant support.
Ziziphora tenuior is a fragrant herbaceous plant whose range covers the territory of Iran. It belongs to the family Lamiaceae and has small leaves and white, pink and purple flowers. Plants of the Lamiaceae family as a whole are of particular interest due to the fact that many representatives of the family have antibacterial, sedative, analgesic and immunostimulating effects [13]. In particular, drugs based on ziziphora have effects such as protection of cardiomyocytes from hypoxia [14].
Evaluation of the effect of Ziziphora tenuior extract on biochemical parameters, including liver function tests and lipid profile, showed that cholesterol and alanine aminotransferase levels decreased in the groups receiving this extract. The main compounds of ziziphora include pulegone, a biologically active compound that is used in the treatment of fever and stomach tone due to its analgesic and anti–inflammatory effects. Ziziphora thin has lipid-lowering properties and improves oxidative stress in the lungs and liver of rabbits. Phytochemical studies have established the presence in the composition of the corresponding raw materials of six derivatives of flavonoids, luteolin, apigenin, 5-O-methylapigenin, apigenin-7-O-glucoside and ziziphorins A and B, as well as some derivatives of triterpenoids, 3', 5'-dihydroxyacetophenone, isomentone, 2-methyl-5-(1-methylethyl) phenol, limonene, 12 acetyl-4, 4 d-methylcyclopentane-2-isene, ziziphorins, anthocyanins, proteins. Steroid compounds, carvacrol, phytonutrients limonene and alpha-terpineol in plants of this family also have analgesic and antibacterial properties, therefore, components based on raw materials of its representatives are used in the food industry as part of dairy products as flavorings and preservatives. Due to their antioxidant activity, extracts from these plants improve oxidative stress in the liver and lungs, increase the activity of antioxidant enzymes in the body and reduce oxidative stress [15].
Using plant extracts with cardioprotective properties in production compound feeds not only reduces the risk of cardiovascular diseases that negatively affect the growth, survival, and quality of reproductive cells in aquaculture facilities. It also provides the body with biologically active substances, antioxidants, and mineral elements. These elements, among other things, improve the activity of the heart in farmed fish.
The search for new patterns of sturgeon food strategy based on the study of the possibility of optimizing the growing process on a feed formulation with the desired components, determined the purpose of the study – to study the fish-breeding and physiological-biochemical parameters of sterlet when using Leonurus cardiaca and Ziziphora tenuior in feeding.
Material and methods
Experimental work was conducted in 2023 at Astrakhan State Technical University. The research object was a Sterlet (Acipenser ruthenus Linnaeus, 1758), weighing 100 grams each and in its second year of cultivation. It was imported from a cage enterprise in the Astrakhan Region in the spring. In total, 24 healthy individuals of the same size were randomly divided into 3 fish breeding tanks with a capacity of 400 liters and
a planting density of 8 fish per tank. Fish feeding was carried out according to the following scheme: control group and experimental groups 1 and 2.
The experiment lasted 30 days, starting with a 10-day period of adaptation for the fish in the tanks. The fish were fed a diet of our own formulation, which included common ingredients such as fish flour, meat and bone meal, gluten, pumpkin cake, oat and wheat flour, soleros, soy flour, feed yeast, probiotic, premix, and fish oil. Additionally, the diet contained various concentration additives: option 1 included motherwort at 1%, option 2 included ziziphora at 1%, and there was a control group without any additives.
The proposed components in the formulations, including those containing phytomeans, were ranked by percentage in accordance with the formulation, the resulting mixture was thoroughly mixed, the prepared wet mixture of experimental compound feeds was formed in the form of cylindrical filaments with a diameter of 3.5 to 7.5 mm, after which it was dried with warm air at a temperature of 55.5 °C in a universal drying cabinet “Ekros” PE-4610 series. The dried feed pellets were ground to the required size. Feed pellets after preparation and spraying of fat – containing components on them in accordance with the formulation complied with the requirements of SS 10385-2014 “Compound feed for fish” and looked as follows – the finished product in the form of hard grits, the surface is matte, pale brown color without cracks, as well as crumbliness, water resistance and passage of compound feed through a sieve corresponded to the above SS. The nutritional value of the compound feed was calculated according to the developed methods [16]; the physico-chemical composition of the compound feed corresponded to SS 10385-2014 p. 5.3.4, the percentage of animal and vegetable proteins corresponded to the nutritional needs of sturgeon. New formulations were tested to determine the percentage of crude components: protein according to SS 32044.1 – 2012; fat according to SS 1346.15 – 2016, item 9; ash according to SS 26226 – 95, item 1; fiber according to SS 31675 – 2012, item 6.
Each diet was distributed in parallel fish tanks. The fish were hand-fed according to their feedability at 9:00 and 16:00 every day for 30 days. Filtered water was supplied to each container, covering the consumption. During the entire feeding experiment, the water temperature was maintained at 24.0 ± 2.0 °C, and the oxygen content in the water was 8.0 mg/l. The concentration of ammonia nitrogen was controlled at a level of less than 0.2 mg/l during the entire period.
At the end of the feeding experiment, the individuals were not fed for 24 hours before sampling. The amount of feed consumed in each tank was calculated, and the number and total weight of fish in each tank were determined to calculate parameters related to growth indicators. Objects from each container were measured (body length, body weight, mass of internal organs) for subsequent analysis of fish-breeding and biological indicators.
Using pre-defined size – weight indicators, such as initial and final weights, a number of derived characteristics and coefficients were calculated:
Absolute gain (WG, g) = Final weight (g) – Initial weight (g);
Weight gain coefficient (WGR, %) = ((WG, g) / (Initial weight, d)) · 100;
Average daily increase (WG, g/day) = (Final weight (g) – Initial weight (g)) / number of days;
Average daily growth rate (WG, %) = ((Final weight (g) / Initial weight (g))1/t – 1) · 100 %;
Mass accumulation coefficient (FMA) = ((Final weight (g)1/3 – Initial weight (g)1/3) · 3) / day;
Fulton coefficient (FQ) = Final weight (g) · 100 / Final length (cm)3;
Cardiosomatic Index (CIF) = (Heart mass (mg) / Fish weight (mg)) / 1 000 ‰;
Survival rate (SR, %) = (Final number of fish / Initial number of fish) · 100;
Feed Conversion Rate (FCR) = Amount of feed eaten (g) / (Final weight (g) – Initial weight (g)).
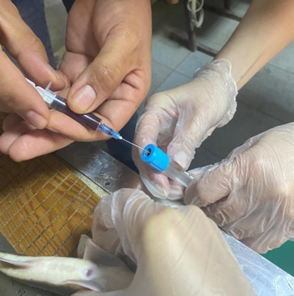
In order to diagnose the condition of objects in each fish tank, blood was taken from the caudal vein from fish using sterile syringes pretreated with a solution of the anticoagulant heparin. The blood taken from each individual was immediately drained into two different Eppendorf–type tubes: one of them contained heparin, which prevents blood clotting, to determine the concentration of hemoglobin; the other was empty, without heparin, to obtain blood serum. Separately, blood samples were taken into vacuum tubes-containers with a coagulation activator for biochemical studies (Fig. 1).
|
а
|
b
|
c
|
|
|
d |
e |
||
Fig. 1. Organization of experimental data collection:
a – determination of dimensional and weight indicators; b – blood sampling from the tail vein using sterile syringes;
c – collection of material for determining the cardiosomatic index; d, e – equipment
For physiological and biochemical blood tests, the concentration of total protein, g/l, the rate of erythrocyte sedimentation (ESR), mm/ h, cholesterol, mmol/l, glucose, mmol/l were determined; among the biochemical parameters of the blood, the level of hemoglobin, g/l was also studied; in serum blood: aspartate aminotransferase (AST), alanine aminotransferase (ALT), mmol/(s·l), cortisol level, ng/ml.
The assessment of the adaptive reaction of the body and the action of the components was carried out according to the indicators of the physiological and biochemical composition of the blood in selected individuals at the beginning and at the end of the growing period. The analysis of the biochemical composition of the blood of cultured fish was performed according to generally accepted methods. The physiological state was assessed according to hematological and biochemical parameters, sampling was carried out in vivo according to recommendations, in compliance with the rules of asepsis and antiseptics (Fig. 1).
The concentration of whey protein in the laboratory was determined by the biuretic method using sets of reagents from the Agat-med company [17], the level of cholesterol in the blood was determined by the enzymatic method using a set of reagents from the Olvexdiagnosticum company [18, 19]. The concentration of glucose in the blood serum was determined by enzymatic colorimetric method without deproteinization (Trinder reaction). A Unico 2100 spectrophotometer was used to measure the optical density of the samples obtained. The concentration of hemoglobin in the blood was determined photometrically using a set of reagents from the Agat-Med company [20], ESR was determined by the Panchenkov method. AST, ALT in blood serum were determined by UV kinetic method, cortisol levels were determined by immunochemiluminescence analysis. Statistical processing. Statistical analysis was performed using statistical functions of the Microsoft Excel and the R language. As the criterion for checking samples in groups for the normality of distribution the Shapiro – Wilk criterion was used. The primary analysis was performed by calculating the main indicators of descriptive statistics, including sample averages and 95% confidence intervals for them, as well as constructing boxplot. The significance level for determining significant differences between the group averages of the pairwise groups was assumed to be 0.05 (differences were considered significant for p < 0.05). To calculate the values of confidence intervals for sample averages, the confidence probability value was assumed to be 95%. The differences in sample averages were evaluated using the ANOVA, and multiple comparison post-hoc procedure was performed using the Tukey’s HSD test. The study complied with official Russian guidelines for the care and use of animals for scientific and educational purposes (Federal Law No. 498-FZ of 12/27/2018).
Results and discussion
For theoretical justification, the possibilities of using natural components of cardioprotectors for fish based on fish-breeding and biological indicators in the composition of feeds have been experimentally confirmed.
The study explored the potential of incorporating natural ingredients from cardioprotective fish-based products into fish feed based on breeding and biological characteristics. The aim was to enhance the health and well-being of fish, particularly sterlet.
The research involved two experimental groups of sterlet fish. The first group served as the control, while the second group received the enhanced feed with the added cardioprotective ingredients.
After conducting the study, it was found that the second experimental group of sterlet individuals outperformed the control group in terms of all breeding and biological indicators. The differences were statistically significant, as shown in Table 1.
Table 1
Fishery-biological indicators of sterlet cultivation
|
Indicators |
Control |
Group 1 |
Group 2 |
|
WG, g |
9.02 |
9.48 |
10.5 |
|
WG, g/day |
0.30 |
0.31 |
0.35 |
|
WG, % |
0.20 |
0.21 |
0.24 |
|
WGR, % |
6.03 |
6.48 |
7.40 |
|
FQ |
0.35 |
0.37 |
0.35 |
|
FMA |
0.0105 |
0.0112 |
0.0126 |
|
FCR |
0.66 |
0.61 |
0.54 |
|
SR, % |
100 |
||
The absolute growth rate exceeded the control by 1.05-1.1 times, the average daily growth rate of 1.05-1.2 times, the gain ratio of 1.07-1.2 times. The feed conversion rate decreased by 8.0 and 22% in the first and second variants, respectively.
Heart. Increased loads on the body of fish, in the case of cultivation in aquaculture conditions, most often caused by a significant deviation from optimal conditions due to the action of certain stress factors; significantly affect the state of the cardiovascular system. The change in physiological parameters is manifested primarily in an increase in heart rate
(a symptom of tachycardia), an increase in respiratory rhythm due to the need to increase oxygen consumption. These symptoms put a strain on the heart and may be the reason for the build-up of its mass. At the same time, there may be changes in the cardiosomatic index within 40%. Thus, in ecologically optimal conditions, the value of the cardiosomatic index is lower than with prolonged exposure to adverse factors. Because of this, the value of this indicator can be used to judge, among other things, the conditions of the facilities and their state of health. The average value of the cardiosomatic index in sterlet of the first and second experimental groups was 1.50 and 1.37, respectively, according to the results of the experiment; it turned out to be 8.05% less in the second group compared with the control (Table 2).
Table 2
Cardiosomatic index indicators
|
Indicators |
Control |
Group 1 |
Group 2 |
|
Cardiac mass, mg |
0.257 ± 0.043 |
0.304 ± 0.048 |
0.259 ± 0.032 |
|
CIF, ‰ |
1.49 ± 0.12 |
1.50 ± 0.08 |
1.37 ± 0.07* |
* The differences are significant at р < 0.05.
Fig. 2 shows a bar chart showing the average values of the CIF in the samples according to the experimental variants.

Fig. 2. Diagram of the value of the CIF, ‰, in each of the groups
For a more detailed analysis of the data obtained for this indicator, a boxplot diagram was constructed (Fig. 3, a), as well as a procedure for analysis of variance, the results of which, adjusted for multiple comparisons using the Tukey’s HSD a posteriori criterion, are presented in Fig. 3, b.
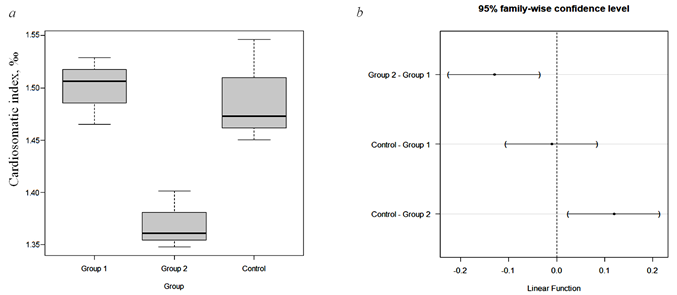
Fig. 3. Visualization of data analysis results for three groups:
a – boxplot for cardiosomatic index in each group; b – ANOVA results, performed by using Tukey’s HSD test
The boxplot diagram illustrates the key statistical features of each group under investigation. The median is represented by a horizontal line, while the upper and lower borders of the rectangles depict the upper and lower quartiles for each sample, respectively. The height of the rectangle indicates the interquartile distance. Additionally, the conditional boundaries of the samples are marked by a dotted line. Any data points located beyond these limits are considered outliers. A preliminary analysis using the boxplot diagram clearly demonstrates that the difference in the values of the cardiosomatic index between the control group and option 2 is most significant. Conversely, the difference for option 1 is minimal. These findings are also supported by the results of analyzing sample averages using analysis of variance with the post-hoc Tukey’s test (Tukey’s HSD) – all-pairs test for multiple comparison procedure. The results in Fig. 3, a illustrate the differences in average values between each pair of compared groups. The groups being compared are indicated on the vertical axis. A 95% confidence interval is calculated for these differences in average values. If the zero difference falls within the boundaries of this confidence interval, it means that there is no statistically significant difference between the groups. In other words, the H0 hypothesis, which states that there are no significant differences, is accepted. However, if the zero difference is not included in the confidence interval, it suggests that there is a statistically significant difference between the groups at a p-value of less than 0.05.
The analysis results shown in Fig. 3, b demonstrate that there are no statistically significant differences between the average values of the cardiosomatic index for the control group and option 1. However, in the case of option 2, statistically significant differences were confirmed. Considering the absolute values of the average sample, it can be argued that option 2 has recorded a statistically significant decrease in the cardiosomatic index (by 8.05% compared to the control). This indicates that a diet with the addition of Ziziphora vulgaris at a concentration of 1% improves the quality of sterlet yearlings and ensures more efficient cultivation.
The data on the cardiosomatic index confirm the significant level of cardioprotective effect of this supplement.
Analysis of blood parameters. To assess the overall health status of the sturgeon fish and detect the potential development of various diseases, a comprehensive analysis of their blood was performed. This included physiological, biochemical, and hematological examinations. The physiological and biochemical parameters of the sturgeon fish that were fed with the proposed diets were not always within their optimal reference ranges. For example, an imbalance in the nutritional components of the compound feeds, such as low protein content, can lead to a deficiency in the diet. This imbalance affects the proportion of hemoglobin in the blood, resulting in a decrease in its level, which can cause anemia in aquaculture facilities. The monitoring of the blood parameters obtained from the control group of sturgeon fish showed minor deviations from their physiological norm (see Table 3).
Table 3
Physiological and biochemical parameters at the end of cultivation
|
Indicators |
Norm |
Control |
Group 1 |
Group 2 |
|
Hemoglobin, g/l |
45.0-75.0 |
53.77 ± 4.39 |
61.77 ± 7.37 |
77.58 ± 11.57* |
|
Total protein, g /l |
20.0-60.0 |
17.34 ± 1.59 |
20.14 ± 1.15* |
24.68 ± 2.37* |
|
ESR, mm/h |
3.0-6.0 |
4.00 ± 0.52 |
1.50 ± 1.16* |
2.83 ± 1.26* |
|
Cholesterol, mmol/l |
1.0-5.2 |
1.69 ± 0.27 |
1.87 ± 0.46 |
1.84 ± 0.35 |
|
Glucose, mmol/l |
1.0-6.0 |
3.49 ± 0.57 |
3.33 ± 0.40 |
2.70 ± 0.62* |
|
AST, μmol/(s · l) |
1.017-2.933 |
0.083 ± 0.006 |
0.18 ± 0.016* |
0.17 ± 0.022* |
|
ALT, μmol/(s · l) |
0.10-0.38 |
0.075 ± 0.019 |
0.226 ± 0.023* |
0.287 ± 0.054* |
|
Cortisol, ng/dl |
10-40 |
14.55 ± 0.91 |
11.15 ± 0.69* |
16.71 ± 1.07* |
|
De Ritis ratio |
0.9-1.73 |
0.91 |
1.26 |
1.68 |
* The differences are significant at p < 0.05.
In the experimental group, there was an increase in hemoglobin levels from 61.77 to 77.58 g/l. This corresponds to an increase of 1.1 to 1.4 times compared to the control group. The observed hemoglobin levels are within the normal range, which eliminates the risk of hypoxia and heart problems.
The Fig. 4 and 5 show the results of the experiment regarding the total protein content in blood serum. The designations in these figures are similar to those in
Fig. 3, a, b.
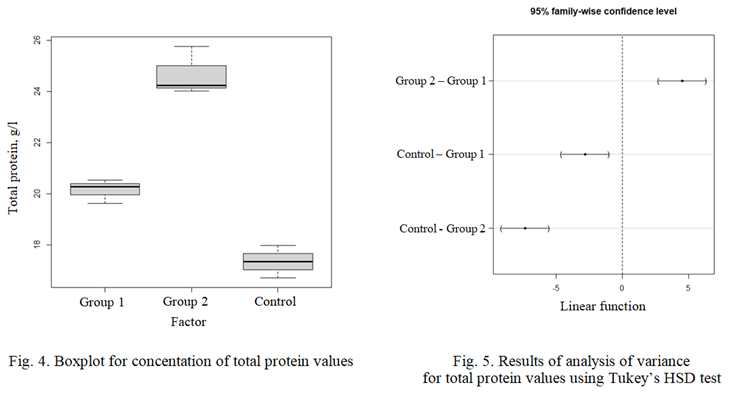
The calculated interquartile distances and sample boundaries indicate significant differences in the indicator between the control group and both experimental groups.
The analysis of variance confirms that there are statistically significant differences between the control group and the experimental groups at a significance level of p < 0.05.
It should be noted that in both experimental groups, the total protein concentration was higher than in the control group: by 16% in the first group and by 42% in the second group. These findings confirm that the conditions for keeping the fish in the first and second experimental groups are optimal or at least much closer to optimal. They also indicate a high level of adaptive capabilities of the fish in these groups.
The protein content in the control group was recorded at the lower limit of the physiological norm (20 g/l). This level is proportional to the level of stress and is likely related to the quality of the tested feed.
Looking at the table, we can see that the erythrocyte sedimentation rate (ESR) is higher in the control group. This may indicate some inflammatory processes in the fish's body, which were more intense in these individuals. In option 1, the corresponding value of the indicator is below the norm – 1.50 mm/h. The second group of studied individuals had ESR values close to the norm, averaging 2.83 mm/h.
The ALT boxplot diagram (Fig. 6) shows essential differences between the control and experimental groups. This finding is supported by the results of the analysis of variance (Fig. 7), which confirms that differences between the average values of the samples are significant (p < 0.05).
|
|
|
|
Fig. 6. Boxplot for ALT values |
Fig. 7. Analysis of variance |
These differences were observed in both cases: when using a feed variant containing motherwort and when using a feed with an additive in the form of ziziphora.
The boxplot diagram for the AST indicator (Fig. 8) also demonstrates a significant difference between the control and experimental samples. The analysis of variance (Fig. 9) reveals statistically significant differences between the control and experimental groups, as well as among the experimental groups themselves.
|
|
|
|
Fig. 8. Boxplot for AST values |
Fig. 9. Analysis of variance of AST using Tukey’s HSD test
|
For ALT, the highest value of the indicator was recorded for the first experimental group, although it does not differ statistically significantly from the similar indicator of the second experimental group. On the other hand, for AST, the highest value was observed in the second experimental group.
It is worth noting that both indicators showed an increase compared to the control group. Moreover, the de Ritis ratio (AST/ALT ratio) in the control group was at the lower limit of the normal range. In contrast, in the first and second experimental groups, this ratio increased, with the second experimental group approaching the upper limit of the normal range.
At the beginning of the experiment, the levels of two enzymes were measured: alanine aminotransferase (ALT) and aspartate aminotransferase (AST). The ALT level was 0.159 ± 0.019 μmol/(s · l), and the AST level was 0.182 ± 0.02 μmol/(s · l). These values were used to calculate the de Ritis coefficient, which came out to be 1.145.
By the end of the experiment, in the control group, this coefficient had dropped to 0.91, which was at the lower limit of the physiological norm. According to the authors, this is a sign of an unfavorable course of pathology related to liver function. It also suggests that the quality of the feed used was poor.
In contrast, the first and second experimental groups showed optimal values. Their ALT levels were 2 times higher than in the control group (0.318 ± 0.035 μmol/(s · l) and 0.345 ± 0.032 μmol/(s · l) respectively). Their AST levels were also higher, but within the normal range. The first experimental group had an AST level that was 3 times higher than in the control group, while the second group had an AST level that was 3.8 times higher. This proves that Leonurus cardiaca has pronounced cardioprotective functions.
The first experimental group had the lowest cortisol index, which suggests that the motherwort supplement has a strong sedative effect.
Compound feed. The expected increase in capacity for the production of aquaculture products makes it necessary to solve the related problem of increasing the production of domestic aquaculture feeds with
a high level of involvement in the turnover of the local raw material base, including to ensure independence from imports, partially or completely stopped under severe sanctions pressure. The lack of feed is currently one of the main factors limiting the development of aquaculture [21]. To an even greater extent, the problem of providing feed of domestic production is significant for enterprises that provide themselves with feed independently, making small batches from raw materials presented on the domestic market.
Despite the fact that the quality of the components was confirmed by quality certificates, the actual study of ready-made compound feeds showed their low quality as part of each of the formulation options.
During the analysis of the nutritional properties of compound feed according to its own developed formulation, a conclusion was made about the low quality of the feedstock (Table 4), despite the fact that quality certificates have been obtained for the incoming components.
Table 4
Nutritional value of experimental compound feeds
|
Weight proportion, % |
Value |
Method of testing |
||
|
Norms* |
Projects values |
Experienced compound feed |
||
|
Crude protein |
42.0 |
43.27 |
23.1-24.4 |
Standard 32044.1 – 2012 |
|
Crude fat |
12.0 |
10.33 |
9.35-9.83 |
Standard 1346.15 – 2016 |
|
Crude ash |
10.0 |
9.5 |
28.0-29.0 |
Standard 26226 – 95 |
|
Crude cellulose |
3.0 |
4.7 |
2.9-2.3 |
Standard 31675 – 2012 |
* Standard 10385-2014.
When experimentally using the obtained compound feed without compensatory additives, a low content of whey protein, a high index of the cardiosomatic index and low activity of the ALT enzyme were noted, which negatively affected the De Ritis ratio and the intensity of biochemical processes in the liver. On the contrary, the use of compound feeds with cardioprotective additives had a positive effect [11], which is reflected in Table 5.
Table 5
Fishery-biological indicators and physiological-biochemical effects
|
Plant ingredients |
Fishery-biological effects |
Physiological-biochemical effects |
|
|
Leonurus cardiaca |
WG, g |
+5% |
Hemoglobin, g/l +14% Blood serum protein content, g/l +16% De Ritis ratio +39% |
|
WG, % |
+3% |
||
|
WGR, % |
+7.5% |
||
|
FCR |
–7.6% |
||
|
CIF, ‰ |
0% |
||
|
Ziziphora tenuior |
WG, g |
+16% |
Hemoglobin, g/l +44% Blood serum protein content, g/l +42% De Ritis ratio +85% |
|
WG, % |
+12% |
||
|
WGR, % |
+22.7% |
||
|
FCR – |
18.2% |
||
|
CIF, ‰ |
–8.05% |
||
Conclusion
Cardioprotective additives of natural origin enhance the digestibility of feed components, leading to improved fish product quality. The research revealed that when feeding sterlets weighing 100 grams with the developed feed and rationing the daily feed amount to 4% of body weight, adding Ziziphora tenuior to the feed formulation at a 1% content level significantly improves heart condition indicators. This experimental formulation has promising potential as a preventive feed. Additionally, when motherwort (Leonurus cardiaca) is added to the feed, the corresponding additive exhibits a sedative effect, as indicated by a decrease in cortisol levels in farmed individuals.
1. Garseth A. H., Fritsvold C., Svendsen J. C., Bang Jensen B., Mikalsen A. B. Cardiomyopathy syndrome in Atlantic salmon Salmo salar L.: a review of the current state of knowledge // J. Fish Dis. 2018. N. 41. P. 11–26.
2. Brijs J., Hjelmstedt P., Berg C., Johansen I. B., Sundh H., Roques J. A. C., Ekstrom A., Sandblom E., Sundell K., Olsson C., Axelsson M., GrÃns A. Prevalence and severity of cardiac abnormalities and arteriosclerosis in farmed rainbow trout (Oncorhynchus mykiss) // Aquaculture. 2020. V. 526. P. 735417. DOI:https://doi.org/10.1016/j.aquaculture.2020.735417.
3. Семыкина А. С., Васильев А. А., Григорьев В. С. Анализ биохимических показателей крови ленского осетра при использовании в кормлении препарата «Виусидвет» // Аграр. науч. журн. 2018. № 8. С. 43–46.
4. Farrell A. P. Coronary arteriosclerosis in salmon: growing old or growing fast? // Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002. V. 132 (4). P. 723–735. DOI:https://doi.org/10.1016/s1095-6433(02)00126-5. PMID: 12095858.
5. Gamperl A. K., Farrell A. P. Cardiac plasticity in fishes: environmental influences and intraspecific differences // J. Exp. Biol. 2004. V. 207. P. 2539–2550.
6. Seierstad S. L., Svindland A., Larsen S., Rosenlund G., Torstensen B. E., Evensen Ø. Development of intimal thickening of coronary arteries over the lifetime of Atlantic salmon, Salmo salar L., fed different lipid sources // J. Fish Dis. 2008. V. 31. P. 401–413.
7. Ekström A., Gräns A., Sandblom E. Can’t beat the heat? Importance of cardiac control and coronary perfusion for heat tolerance in rainbow trout // J. Comp. Physiol. B. 2019. V. 189. P. 757–769.
8. Евграфова Е. М., Пятикопова О. В., Бедрицкая И. Н., Яковлева Е. П., Дубовская А. В., Тангатарова Р. Р., Перунова М. Е. Индексы физиологических признаков белуги и шипа и их межвидовых гибридов в условиях бассейнового хозяйства // Вестн. Астрахан. гос. техн. ун-та. Сер.: Рыбное хозяйство. 2020. № 4. С. 154–164.
9. Mercier C., Aubin J., Lefrancois C., Claireaux G., Cardiac disorders in farmed adult brown trout, Salmo trutta L. // J. Fish Dis. 2000. V. 23. P. 243–249.
10. Poppe T. T., Johansen R., Gunnes G., Tørud B. Heart morphology in wild and farmed Atlantic salmon Salmo salar and rainbow trout (Oncorhynchus mykiss) // Dis. Aquat. Org. 2003. V. 57. P. 103–108.
11. Hamad H. A., Nguyen T. H. V., Kuzmina E. G., Martyanov A. S. Сompound feed with cardioprotective effect for sturgeon fish // Vestnik of Astrakhan State Technical University. Series: Fishing industry. 2023. N. 3. P. 57–65.
12. Исмаилова Ф. О., Гусейнова А. А., Бекшоков К. С. Сравнительное фармакохимическое изучение валерианы лекарственной, пустырника пятилопастного и пиона уклоняющегося // Вестн. Дагестан. гос. ун-та. 2012. № 1. С. 215–219.
13. Tenuior Yang W. J., Liu C., Gu Z. Y., Zhang X. Y., Cheng B., Mao Y., Xue G. P. Protective effects of acacetin isolated from Ziziphora clinopodioides Lam. (Xintahua) on neonatal rat cardiomyocytes // Chin. Med. 2014. Dec 17. V. 9 (1). P. 28.
14. Tian S., Shi Y., Zhou X., Ge L. Total polyphenolic (flavonoids) content and antioxidant capacity of different Ziziphora clinopodioides Lam extracts // Pharmacogn. Mag. 2011. V. 7. P. 65–68.
15. Karimi I., Hayatgheybi H., Motamedi S., Naseri D., Shamspur T. Chemical composition and hypolipidemic effects of an aromatic water of Ziziphora tenuior L. in cholesterol-fed rabbits // JABS. 2013. V. 7. P. 61–67.
16. Пономарев С. В., Грозеску Ю. Н., Бахарева А. А. Корма и кормление рыб. М.: Моркнига, 2014. 465 с.
17. Филиппович Ю. Б., Егорова Т. А., Севастьянова Г. А. Практикум по общей биохимии. М.: Просвещение, 1975. 318 с.
18. Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor // Clinic Chemistry Acta. 1969. V. 6. P. 24–25.
19. Fishbach F. A., Dunning M. A manual of laboratory and diagnostic tests. Philadelphia: Williams & Wilkins, 2004. P. 1291.
20. Van Kampen E. J., Zjilstra W. G. Standardization of hemoglobinometry // Clinic Chemistry Acta. 1961. N. 6. P. 538–544.
21. Лагуткина Л. Ю. Перспективное развитие мирового производства кормов для аквакультуры: альтернативные источники сырья // Вестн. Астрахан. гос. техн. ун-та. Сер.: Рыбное хозяйство. 2017. № 1. С. 67–78.