Россия
Рассматривается опыт применения астаксантина в кормлении стерляди. Изучение влияния астаксантина на морфофизиологическое состояние рыб позволяет разработать высокоэффективные рецепты комбикормов для ценных видов аквакультуры. Данный мощнейший антиоксидант оказывает огромное влияние на ра-боту всех систем и общее состояние здоровья, но такая практика применения астаксантина в кормлении не коснулась ценных объектов аквакультуры. Влияние астаксантина на физиологический статус осетровых рыб до сих пор не изучен. Установлено, что в варианте 2 (добавление 40 мг астаксантина на 1 кг корма) наблюдались самый высокий абсолютный прирост, среднесуточный прирост, среднесуточная скорость роста и коэффициент массонакопления (р ˂ 0,001) в отличие от варианта 1 (добавление 20 мг астаксантина на 1 кг корма) и контроля. Абсолютный прирост в данной выборке был достоверно выше контрольной в 2,3 раза. Среднесуточная скорость роста в данном варианте составила 1,8 %, что достоверно выше контрольной группы в 2 раза (р ˂ 0,001). Полученные данные свидетельствуют об эффективном действии астаксантина (40 мг/кг) на рыбоводно-биологические показатели культивируемых рыб. Показатели энергетического обмена также свидетельствуют о лучшем накоплении пластических веществ у рыб экспериментальных групп (варианты № 1 и 2): гемоглобин в вариантах с добавлением астаксантина в корма составил 77,4 и 71,3 г/л, что на 17,6 и 8,4 % выше, чем у рыб контрольной группы. В экспериментальных вариантах в сравнении с контрольной выборкой уровень общего белка был достоверно выше на 17,4 и 22,2 % (р ≤ 0,05). Таким образом, можно говорить, что антиоксидант астаксантин обеспечил более благоприятные трофические и биохимические условия, необходимые для нормального роста и развития рыб.
антиоксидант, астаксантин, аквакультура, кормление, стерлядь, прирост, физиолого-биохимические показатели
Introduction
The world production of fish and seafood was growing steadily in the foreseeable past (the FAO statistical series began in 1950) and is increasing today. At the same time, fish farming is gradually replacing industrial fishing: the global catch of fish has been approximately constant since the mid-1980s, but at the same time the production of aquaculture products is advancing. Almost the entire increase in global fish consumption over the past 30 years is stipulated by growth in the sector of breeding and cultivation of aquatic organisms. This industry has a great future, unlike traditional industrial fishing, which, of course, will continue to play an important role in supplying humanity with fish and seafood [1].
The increasing production of aquaculture products proves the significance of feed production to provide the industry with high-quality compound feeds and feed additives that meet modern requirements and biological needs of the cultivated fish. The development of aquaculture requires special attention to the feeding process and the use of full-fledged and environmentally safe feeds for all objects of aquacultures [2]. In the process of developing the combined feed recipes, in addition to the balance in basic nutrients, much attention is paid to the adequate use of various biologically active substances, which include natural pigments carotenoids [3]. They play a different role in the fish metabolism and are potent antioxidants as well. Carotenoids protect the body from the impact of free radicals, which damage the membranes of fish cells. Astaxanthin is a xanthophyll carotenoid, a natural substance that is quite widespread in nature. Astaxanthin shows higher activity than other antioxidants, because due to its chemical structure it binds the inner and outer cell membranes. Astaxanthin is 550 times stronger than vitamin E and 6 000 times stronger than vitamin C. In addition, it is 10 times more effective than zeaxanthin, lutein, canthaxanthin and various forms of beta-carotene. The research aims to investigate natural astaxanthin, its antioxidant, provitamin and antimutagenic activity, its use in the food industry, agriculture and medicine [4]. The purpose of the work was to study the effectiveness of using astaxanthin in composition of feed for the valuable aquaculture objects.
The set goal defined the following tasks:
– to evaluate the results of sterlet cultivation on production compound feeds with the addition of astaxanthin by the fish-breeding and biological parameters;
– to assess the physiological state of the cultivated fish.
Materials and methods of research
Experimental work was carried out on the basis of the Innovation Centre “Bioaquapark – Research Technological Centre of Aquaculture” under Astrakhan State Technical University. The objects of the study were sterlet yearlings. There was studied the effectiveness of using the natural antioxidant astaxanthin, trade name “Astaped” (manufactured in India) (Fig. 1).

Fig. 1. Antioxidant astaxanthin
The bioavailability (ability to be absorbed by the body) of astaxanthin is not too high, but the absorption of astaxanthin improves when combined with edible oils, for example, fish oil. Astaxanthin is a lipophilic compound, it dissolves well in oils. Before being introduced into experimental feeds, astaxanthin was dissolved in liquid fish oil.
The study was carried out in three experimental groups. The first group (control) received a food product balanced in all nutrition elements, according to the physiological needs. The second group (test No. 1) received the diet of the 1st group with the natural antioxidant astaxanthin in the amount of 20 mg/kg. The third group (test No. 2) received the diet of the 1st group with the natural antioxidant astaxanthin in the amount of 40 mg/kg (Table 1).
Table 1
The main parameters of the experiment
|
Indicators |
Variants of tests |
||
|
Control |
Test No. 1 |
Test No. 2 |
|
|
Diet |
Main diet (MD) |
MD + 20 mg/kg astaxanthin |
MD + 40 mg/kg astaxanthin |
|
Pellet size, mm |
2.0 |
||
|
Tanks |
ITSA – 1 |
||
|
Stocking density |
25 pcs/m2 |
||
|
Methods of feeding |
Manually, by eatability |
||
|
Water temperature, °С |
21.4 ± 0.46 |
21.5 ± 0.18 |
22.0 ± 0.60 |
|
рН, units |
7.5 |
||
|
Research period |
40 days |
||
|
Survival rate, % |
100 |
||
Experimental feed was produced in the laboratory conditions by wet pressing method using feed components produced in Russia. Fish feeding was carried out manually 2 times in the daytime. The daily feeding rate was determined according to the feed tables depending on the fish average weight and water temperature [2].
Cultivation was carried out at the similar planting density and constant temperature regime in accordance with the biological characteristics of the species.
The condition and development of fish was determined by different indicators analyzing the rate of increase in body size and muscle mass building. Weighing and measuring of fish was carried out according to the recommendations adopted in fish farming using laboratory scales Massa-K VK-3000 [5].
Survival rate of fish was taken into account by the single species method. Physiological state of the studied objects was assessed by the biochemical parameters of protein, lipid and carbohydrate metabolism (blood composition), according to the developed methods [6-9]. Blood was taken in vivo from the tail vein of fish into Eppendorf tubes [10]. The following factors were determined: concentration of hemoglobin photometrically using reagents from Agat-Med [7], erythrocyte sedimentation rate (ESR) by using the gauge of R. P. Panchenkov [10]. A Unico 2 100 spectrophotometer was used to measure the optical density of the samples obtained. The results of the research were processed using generally accepted methods
of biological statistics and the Microsoft Excel program. The level of differences was assessed by applying the Student's reliability criterion [11].
Research results
Dynamics of sterlet growth on tested experimental feeds is shown in Fig. 2.
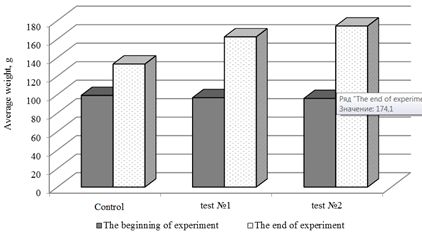
Fig. 2. Dynamics of sterlet growth on experimental feeds
Evaluation of the effectiveness of using astaxanthin in compound feeds production showed that the best growth rates were found in a group of fish that consumed feed with the addition of astaxanthin 40 mg per 1 kg of feed (test No. 2). In test No. 2, the highest absolute growth, average daily growth, average daily growth rate and mass accumulation coefficient (p < 0.001) were observed (Fig. 3).
|
a |
b |
Fig. 3. Piscicultural indicators of sterlet cultivated on experimental feeds: a – absolute growth;
b – mass uccumulation coefficient, units
Absolute increase in this group was for certain 2.3 times higher than in the control one. The average daily growth rate in this test was 1.8%, which is for certain 2 times higher than in the control group (p < 0.001). The data obtained show the positive effect of astaxanthin (40 mg/kg) on the fish–breeding and biological parameters of the cultured fish.
In test No. 1 fish consumed feed with astaxanthin proportion of 20 mg/kg, its average daily growth rate was 94.1% higher than that of the control. The absolute increase in live weight in fish of the first group was 65.8 g versus 78.3 g in the second group, which was 1.9 and 2.3 times higher than in fish of the control group.
The mass accumulation coefficient in fish in the first variant was 0.06 units and in the second 0.07 units, which is 0.03 and 0.04 units, respectively, higher than in the control. The average daily increase in variants 1 and 2 was: 1.65 and 1.96 g, which is 1.9 and 2.3 times higher, respectively, then in the control group. The survival rate in the experimental variants and control was 100%. It was found that the best growth rates were registered in test No. 2, in fish that consumed feed with astaxanthin proportion of 40 mg per 1 kg of feed. This is quite understandable, according to the literature, the antioxidant astaxanthin has a unique molecular structure that allows it to be both inside and outside the cell membrane at the same time. The protection mechanisms
of astaxanthin against oxidative damage include suppression of singlet oxygen, absorption of radicals to prevent chain reactions, inhibition of peroxidation to preserve the membrane structure, improvement of immune system function and regulation of gene expression [4]. Thus, adding the antioxidant astaxanthin to the feeds contributes to the increasing productivity, fish have better fish-breeding and biological parameters, which contributes to the fish weight gain. As a result, the inclusion of the antioxidant astaxanthin in the feeds has contributed to a more efficient use of nutrients in the diet. One of the elements of the biochemical assessment of the physiological state of cultured fish is the characteristic of the metabolic function of blood [12]. In order to identify changes in metabolic processes, the dynamics of such indicators as ESR, concentration of hemoglobin, serum protein, glucose, cholesterol, beta-lipoproteins and albumin in blood serum were studied. The data on physiological indicators of juveniles in natural conditions were used as normative values: ESR – 2.0-4.0 mm/h, hemoglobin – 50.0-80.0 g/l, cholesterol – 1.0-3.5 mmol/l, total protein – 20.0-40.0 g/l, beta-lipoproteins – 1.0-5.0 g/l, glucose – 3.0-6.5 [13]. The results of analysis of the physiological state of the sterlet are presented in Fig. 4.
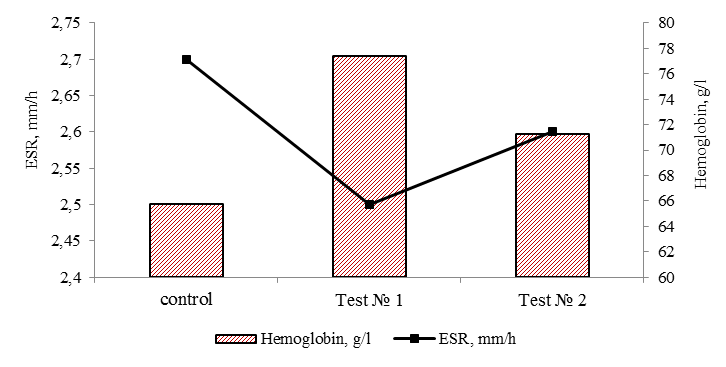
Fig. 4. Hematological parameters of sterlet blood at the end of the experiment
Sedimentation rate of erythrocytes and hemoglobin in all variants of the experiment remained within the normative values, which indicates a constant protein composition of blood plasma. According to the ESR indicator, no differences were found in the study variants (p > 0.05).
As can be seen in Fig. 4, there is some positive dynamics in using astaxanthin in tests No. 1 and 2, the hemoglobin levels increase. Hemoglobin in tests No. 1 and 2 was 77.4 and 71.3 g/l, which is 17.6 and 8.4% higher than in the fish of the control group. High concentration of hemoglobin in the experimental groups (within the reference values) may be associated with a more intensive metabolism in the fish body due to the antioxidant astaxanthin used in the feeds, which increases the rheological parameters of the blood, which suggests a positive effect on the microcirculation of blood in the fish body. It is difficult to underestimate such property of astaxanthin [4]. The blood circulation depends on how well the organs are supplied with nutrients and oxygen.
The leukocyte blood formula, which reflects not only the physiological state of fish, but also some aspects of cellular immunity, is a fairly informative indicator in assessing the overall physiological state of the body. Changes in the leukogram can detect metabolic disorders and deterioration of the studied object long before the manifestation of clinical signs of emerging pathologies [14]. The leukocyte formula of the blood of the fish under study is presented in accordance with Fig. 5.
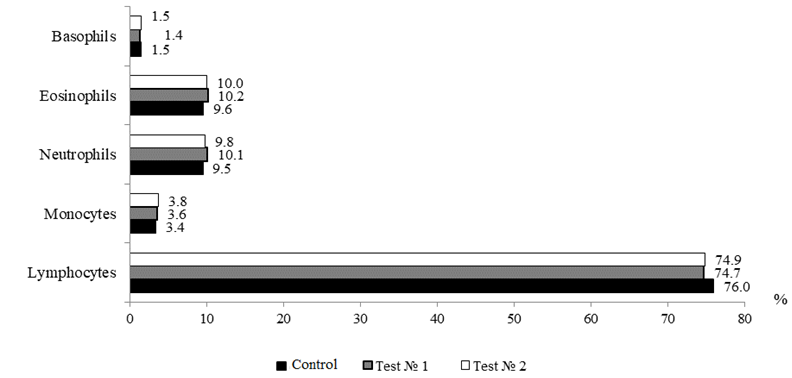
Fig. 5. Ratio of shaped blood elements in sterlet
Fish are characterized by a lymphocytic profile where the base of white blood is presented by lymphocytes, the proportion of which in the total pool of leukocytes does not fall below 70% and can exceed 90%. Lymphocytes are responsible for immune surveillance, formation and regulation of cellular and humoral immune response, synthesizing protective antibodies, lysing foreign cells, ensuring the destruction of their own mutant cells. As can be seen from Fig. 5, the proportion of lymphocytes in the studied groups ranged within 74.9-76.0% (p > 0.05), these figures correspond to the physiological norm for fish. Leukocyte shifts indices in the experimental variants ranged from 0.26 to 0.28%, these indicators were within the reference values
(0.25-0.40% for sturgeon fish).
The dynamics of biochemical indicators serves as a marker of the state of the fish organism in artificial and natural reservoirs, characterizes the quality and quantity of nutrition, planting density, adaptive abilities of fish and intensity of anthropogenic factors [15, 16]. The results of the biochemical study are presented in Table 2.
Table 2
Physiological and biochemical parameters of sterlet blood
(numerator – beginning of the experiment, denominator – end of the experiment) (n = 25)
|
Indicator |
Control |
Test No. 1 |
Test No. 2 |
|
Total protein, g/l |
28.5 ± 1.5 28.8 ± 9.5 |
21.6 ± 0.9 35.2 ± 1.6* |
24.6 ± 0.6 33.8 ± 8.7* |
|
Glucose, g/l |
2.2 ± 0.2 2.5 ± 0.8 |
2.6 ± 0.7 3.0 ± 0.6 |
2.5 ± 0.2 3.6 ± 0.5* |
|
Cholesterol, mmol/l |
3.3 ± 0.2 3.5 ± 0.2* |
3.5 ± 0.3 2.4 ± 0.2 |
3.4 ± 0.2 2.0 ± 0.1 |
|
Beta-lipoproteins, g/l |
3.3 ± 0.2 3.5 ± 0.3 |
3.0 ± 0.3 3.3 ± 0.2 |
2.8 ± 0.4 3.5 ± 0.3 |
|
Albumin, g/l |
24.9 ± 5.1 31.6 ± 0.3 |
22.0 ± 4.3 32.0 ± 1.2 |
25.8 ± 0.4 33.0 ± 1.2 |
* P < 0.05 – the differences are significant.
The level of total whey protein at the beginning of the study for tests No. 1 and 2 was low, but it was within the normative values and relatively homogeneous. Variability was 1.5-4.0%. The obtained low data on the concentration of total protein in the blood before the start of the experiment indicate a high level of stress load, which can be overcome due to the additional energy obtained in the course of protein disintegration. At the end of the experiment, the indicators of total protein were for certain higher in tests No. 1 and 2 by 17.4 and 22.2% compared with the control sample. Such dynamics corresponds to the data on the growth rate. At the end of the experiment in tests No. 1 and 2 the glucose dynamics was within the physiological norm (from 3.0-4.0 g/l), which is the result of the normal operation of the enzymatic system that catalyzes the transformation of glucose, the control group was characterized by low glucose concentrations below the reference values.
Cholesterol concentration in the blood plays an important role for the growth of the fish body and cell division; cholesterol comes from food or is produced by the fish own cells or synthesized in the liver. The cholesterol level in the blood of fish above 3.7 g/l is considered pathological and indicates the effect of stressful environmental factors. In the conditions of the study, there was registered a tendency of decreasing cholesterol in the blood of fish that consumed feed with the addition of astaxanthin to the feed formulation. At the same time, the greatest changes occurred in test No. 2 (70.0%), while in test No. 1 the indicator decreased by 45.8%. In the control variant, the cholesterol level was significantly higher (p < 0.05) and did not even change slightly in comparison with the initial data. The most cholesterol-rich particles are beta-lipoproteins (low-density cholesterol). Under growing conditions, this indicator was at the level of 2.8-3.05 g/l, which corresponds to the values (1.0-5.0 g/l) characteristic for the fish living in the natural habitats [13]. There were no differences in the level of beta-lipoprotein concentration (p > 0.05).
The term ‘total protein’ refers to the total concentration of albumin and globulin in the blood serum. Of all the proteins, albumin synthesized in the liver is present in the highest concentration in plasma. It is necessary
to maintain osmotic balance ensuring the normal distribution of fluid between the blood vessels and the extravascular space. With starvation or insufficient intake of proteins from food, the albumin content in the plasma drops, which can lead to increased accumulation of water in the tissues (edema). This condition associated with protein deficiency is called starvation edema [15]. As a result of the conducted studies, there can bs seen an increase in this indicator after feeding in all experimental variants, any significant differences are not stated among these groups (p > 0.05). Thus, the results obtained indicate the effective utilization of the feed and activation of plastic metabolism, which is confirmed by the data of physiological-biochemical and fish-breeding-biological analysis, as well as the absence of any disturbance in the transformation of substances in the body. This fact indicates the effectiveness of using astaxanthin in sturgeon feed, the highest fish-breeding and biological indicators were shown in test No. 2, where astaxanthin was added to feed in the proportion of 40 mg per 1 kg of feed.
Conclusion
The conducted studies indicate the effectiveness of using astaxanthin in nutrition of valuable aquaculture objects, in particular, sterlet. The positive effect of the natural antioxidant astaxanthin on the growth and development of cultured juveniles has been established. In general, the obtained results of hematological and biochemical parameters are consistent with the data of other authors [12, 13]. The fish during the experiment with astaxanthin did not show any stress signs after feeding. Astaxanthin did not show a pigmenting role in the staining
of sterlet tissues.
Thus, according to the biological indicators of growth and physiological state, we can talk about the effectiveness of adding astaxanthin to sterlet feed in the amount of 40 mg/kg, since this sample is characterized by a high growth rate and higher indicators of protein, carbohydrate and lipid metabolism.
1. Хохлова Н. Ф. Тенденции развития рыбоводства и рыболовства в России // Вестн. Моск. финанс.-юрид. ун-та. 2021. № 4. С. 109-119.
2. Пономарев С. В., Бахарева А. А., Грозеску Ю. Н. Корма и кормление рыб в аквакультуре. М.: Моркнига, 2013. 417 с.
3. Грозеску Ю. Н., Митрофанова М. А. Новый каротиносодержащий препарат с составе комбикормов для осетровых рыб // Вестн. Астрахан. гос. техн. ун-та. Сер.: Рыбное хозяйство. 2004. № 2 (21). С. 81-88.
4. Пономарев С. В. Каротиноиды в аквакультуре осетровых рыб. Ростов н/Д.: Изд-во ЮНЦ РАН, 2010. 148 с.
5. Правдин И. Ф. Руководство по изучению рыб. М.: Пищ. пром-сть, 1966. 376 с.
6. Колб В. Г., Камышников В. С. Клиническая биохимия: пособие для врачей-лаборантов. Минск: Беларусь, 1976. 311 с.
7. Филиппович Ю. Б., Егорова Т. А., Севастьянова Г. А. Практикум по общей биохимии. М.: Просвещение, 1975. 318 с.
8. Fishbach F. A., Dunning M. Manual of laboratory diagnostic tests // Lppincott Williams & Wilkins. 2004. V. 14. Р. 1291.
9. Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor // Clinic Chemistry Acta. 1969. V. 6. P. 24-25.
10. Усов М. М. Морфология и физиология рыб. Лабораторный практикум: учеб.-метод. пособие. Горки: Изд-во БГСХА, 2017. 114 с.
11. Лакин Г. Ф. Биометрия. М.: Высш. шк., 1990. 293 с.
12. Akhmedzhanova A., Evgrafova E., Fedorovykh Y., Lagutkina L., Ponomarev S., Levina O. Bioindicators of homeostasis’ constants of growing conditions of warm-water aquaculture objects in the context of obtaining marketable products // IOP Conf. Series: Earth and Environmental Science. 2021. N. 937. URL: https://iopscience.iop.org/article/10.1088/1755-1315/937/3/032032/meta (дата обращения: 22.11.2022).
13. Матишов Г. Г., Кокоза А. А., Металлов Г. Ф., Гераскин П. П. Комплексный подход к проблеме сохранения и воспроизводства осетровых рыб Каспийского моря. Ростов н/Д.: Изд-во ЮНЦ РАН, 2017. 352 с.
14. Пронина Г. И., Корягина Н. Ю. Методология физиолого-иммунологической оценки гидробионтов. СПб.: Лань, 2017. 96 с.
15. Гулиев Р. А., Мелякина Э. И. Некоторые биохимические показатели крови рыб дельты Волги // Вестн. Астрахан. гос. техн. ун-та. Сер.: Рыбное хозяйство. 2014. № 2. С. 85-91.
16. Камышников В. С. Справочник по клинико-биохимическим исследованиям и лабораторной диагностике. М.: МЕДПрессинформ, 2009. 45 с.