Россия
Россия
Россия
Россия
Приведены результаты исследования влияния минерально-витаминной добавки Е-селен на фи-зиологическое состояние гибрида русско-ленского осетра, не достигшего половой зрелости, и производителей маточного стада гибрида стерлядь × белуга. Для этого изучали комплекс физиолого-биохимических показателей крови (гемоглобин, СОЭ, общий белок, β-липопротеиды, холестерин), отражающих интенсивность и направленность метаболизма, а также состояние гонад по морфологическим признакам с использованием УЗИ-сканирования и анализа гистологических препаратов гонад. Поставлены две серии экспериментов с добавлением препарата Е-селен в корм: опыты с неполовозрелыми рыбами – с низкими (300 мкг/кг) и высокими (2 000 мкг/кг корма) концентрациями – и опыты с половозрелыми гибридами, в корм которых добавляли препарат из расчета 1 мг селена и 100 мг витамина Е / кг корма. Использование препарата Е-селен в первой серии опытов не способствовало интенсификации генеративной функции, что подтверждено уровнем и направленностью обмена веществ, однако оно положительно отразилось на уровне массонакопления у рыб в варианте опыта при высоких концентрациях препарата Е-селен. Применение препарата в кормах половозрелых гибридов стерлядь × белуга выявило положительное его влияние на репродуктивную систему. Повышение интенсивности генеративного об-мена, маркером которого служило содержание в крови общего белка и β-липопротеидов, способствовало увеличению, в сравнении с контролем, доли созревших самок (IV стадия зрелости гонад) в 2 раза и снижению доли самок, оставшихся на II стадии созревания, в 1,8 раза по отношению к контролю. В случае с контролем обнаружены признаки небольшого стрессового состояния рыб, которые в опытной партии рыб не проявлялись.
аквакультура, осетровые виды, витамин Е, селен, репродуктивная функция, кровь, гемоглобин, липиды, общий белок
Introduction
One of the urgent tasks of industrial aquaculture is the normalization of the generative function of females, the development of which occurs in altered aquatic environments that increase the instability of its functioning. This is associated with a somewhat transformed functional state accompanying their maturation [1]. As is known, the habitat and the nutritional completeness of fish diets significantly influence the physiological state of fish, especially during gonadogenesis, when the female body sensitively reacts to all changes in the external environment. Deviations in water parameters from optimal levels lead to additional expenditures of energy substances on the functioning of adaptation mechanisms, while incomplete diets result in prolonged inter-spawning periods. In most cases, commercially produced compound feeds are used for aquaculture fish species, aimed at rapid body mass gain. An alternative approach could significantly increase feed production costs, rendering the process unprofitable. Therefore, in practice, various substances are added to commercial feeds intended for broodstock to enhance their nutritional value and improve fish farming indicators such as physiological state, productivity, immune status, disease resistance, and microbiota composition [2, 3].
Vitamin supplements, such as vitamin E and ascorbic acid [4], also affect fertility. Increasing the dietary content of ascorbyl monophosphate in broodstock led to a significant increase in fertility and offspring survival. The best results were achieved at 870 mg of ascorbyl monophosphate per kg of feed, where maximum reproductive efficiency was observed.
Research on the effects of vitamin supplements has also been conducted on other fish species, generally revealing positive outcomes. For example, supplementing standard granulated feeds for carp broodstock during the pre-spawning period with elevated levels of vitamin A (2 500 and 5 000 IU/kg of feed) increased the working and relative fertility of females and the volume of milt in males, as well as the number of larvae hatched from eggs [5].
Gonadogenesis is associated with increased peroxidation processes in fish during the biosynthesis of steroid hormones, thereby increasing the demand for antioxidants. The antioxidant function of vitamins E and C is well known. Vitamin E is considered the primary fat-soluble antioxidant, acting on cell membranes to control the intensity of free radical reactions. Vitamin C, or ascorbate, like vitamin E, is a direct-acting antioxidant, reacting with hydroperoxyl radicals [6]. Additionally, it can restore the antioxidant properties of fat-soluble vitamin E. Vitamins E and C are synergists. Selenium is a notable synergist for both vitamins, being a component of glutathione peroxidase-an enzyme that protects cells from the negative effects of radicals.
Beyond direct action, vitamin E and selenium can enhance the expression of antioxidant genes. Experiments with broilers fed diets enriched with vitamin E and selenium showed that they not only act as exogenous antioxidants but also induce the expression of endogenous antioxidant enzyme genes as gene regulators [7].
Selenium is one of the primary components of the body's antioxidant defense system. It protects the body from the aggressive effects of free radicals, which can reduce immunity, damage cell membranes, and DNA.
The development of industrial technologies in aquaculture poses challenges for fish farmers to breed highly productive fish, whose genetic potential can be unlocked by adding appropriate biologically active substances to their feed at specific developmental stages. This primarily concerns broodstock, whose diets require higher standards. These supplements can help maintain the reproductive function of breeders at the required level, resulting in healthy offspring with high viability potential.
The aim of this work was to evaluate the influence of various biologically active substances on the optimization of physiological processes in young fish and potential sturgeon broodstock under artificial conditions for reproduction purposes.
To monitor the health of reproductive fish stocks under the influence of feed additives, it is necessary to study the blood characteristics of aquatic organisms [8]. Biochemical blood parameters serve as indicators of the physiological state of fish [9].
Materials and methods
To study the influence of mineral-vitamin feed additives on sturgeon gonadogenesis, two series of experiments were conducted. The first evaluated the effect of the E-selenium supplement on the physiological state of young fish and its influence on the degree of gonad formation in the Russian-Lena sturgeon hybrid (Acipenser gueldenstaedtii Brandt, 1833 × Acipenser baerii Brandt, 1869) that had not reached sexual maturity in a recirculating aquaculture system (RAS) at low and high concentrations of the supplement. This experiment lasted 5 months and involved 120 individuals: 60 in the experimental group and 60 in the control group. The second series examined the effect of this supplement on the direction of metabolism in sterlet × beluga hybrid (Acipenser ruthenus Linnaeus, 1758 × Huso huso Linnaeus, 1758) broodstock in RAS. This experiment lasted 50 days, concluding at the start of artificial wintering, with 15 individuals in the experimental group and 14 in the control group.
In the first case, the Russian-Lena sturgeon hybrid was fed with the production feed Efico Sigma 860 No. 3-4 by BIOMAR, supplemented with E-selenium by spraying at a concentration of 300 μg/kg. In the second case, broodstock were fed granulated feed Coppens 13/50. The E-selenium supplement was added by spraying, diluted in water at a 1 : 50 ratio. The dosage was 2 ml/kg of feed, corresponding to 1 mg selenium and 100 mg vitamin E per kg of feed.
This dosage was selected based on literature data on the biological requirements of fish for selenium (0.15-0.50 mg/kg) and vitamin E (20.0-100.0 mg/kg),
as well as regulatory doses of these components in fish feeds (selenium 0.15-1.50 mg/kg, vitamin E 50.0-100.0 mg/kg) [10, 11]. The control group received feed without E-selenium.
The physiological state of the hybrid individuals was assessed based on blood content of hemoglobin, total serum protein, total cholesterol, total lipids, β-lipoproteins, and erythrocyte sedimentation rate (ESR). Blood was drawn from the caudal vein (vena caudalis).
Erythrocyte sedimentation rate was determined using a PR-3 device. Serum protein concentration was measured with an IRF-454B2M refractometer. Hemoglobin concentration was determined using a reagent kit by PLIVA-Lachema [12]. Total cholesterol was measured with a reagent kit by “Olvex Diagnostikum” [13]. Total serum lipids were analyzed using a PLIVA-Lachema kit, and β-lipoproteins were determined turbidimetrically. The experimental results were processed using standard biological statistics methods and Microsoft Excel. The reliability of differences was assessed using Student's t-test [14].
Results and discussion
The study of the possible positive effects of E-selenium on the Russian-Lena sturgeon hybrid was conducted on two groups of fish with approximately the same initial mass – about 130 g. Over the 5-month experiment, the difference in mass and length between the experimental and control groups was 3%, with higher values in the experimental fish. The main environmental parameters for both groups were similar and within normal ranges. The study of physiological and biochemical parameters during the experiment revealed similar dynamics. For example, changes in hemoglobin content and ESR levels were synchronous in both groups, with no significant differences between the start and end of the experiment (p > 0.05). These parameters increased by about 30% from the start (Hb ~50 g/L, ESR 1.6 mm/hour) to the end (Hb ~64 g/L, ESR 2.2 mm/hour). The content of total protein in the blood (Fig. 1) changed synchronously in both groups, but was significantly lower (p < 0.05) in the experimental group at the end, with higher initial values, likely due to its intensive use for mass gain in the experimental fish.

Fig. 1. Dynamics of total serum protein in the Russian-Lena sturgeon hybrid in the experiment
on the influence of low concentrations of the E-selenium vitamin-mineral supplement
The variability of cholesterol levels in the blood of the studied fish was notable in both the control and experimental groups (Fig. 2).

Fig. 2. Dynamics of cholesterol in the Russian-Lena sturgeon hybrid in the experiment on the influence of low concentrations of the E-selenium vitamin-mineral supplement
Initially, cholesterol levels were higher in the experimental group (p < 0.05), but they equalized over time. The simultaneous increase in cholesterol levels in both groups in May (exceeding 4 mmol/L) suggests that this was unrelated to E-selenium but due to another factor affecting both groups. Notably, in wild sturgeons at sea, cholesterol levels do not exceed 3 mmol/L [15]. The multifunctional role of cholesterol determines its blood concentration, including as a precursor to the stress hormone cortisol.
Total lipid levels in the blood of the experimental and control fish ranged from 2 to 5 g/L throughout the experiment. However, lipid levels were higher in the experimental group. It is believed [16] that E-selenium positively affects lipid metabolism by increasing the concentration of omega-3 polyunsaturated fatty acids, which enhance survival and growth. Low concentrations of E-selenium may only slightly improve overall metabolism, stimulating mass accumulation.
The study of the stimulatory effects of high E-selenium concentrations (2 000 μg/kg feed) on the physiological state of fish was also conducted on the Russian-Lena sturgeon hybrid raised in a RAS under uniform conditions. At the start, the experimental and control groups had similar mass and size indicators, with an average mass of about 230 g (20 g higher in the control). By the end, the mass of the experimental fish increased by 1.7 times, compared to 1.4 times in the control, indicating that E-selenium stimulated metabolism toward muscle mass accumulation.
As in the low-concentration experiment, protein utilization for mass gain was higher in the experimental group, leading to a 1.2-fold decrease in protein levels by the end, compared to 1.1-fold in the control (Fig. 3).
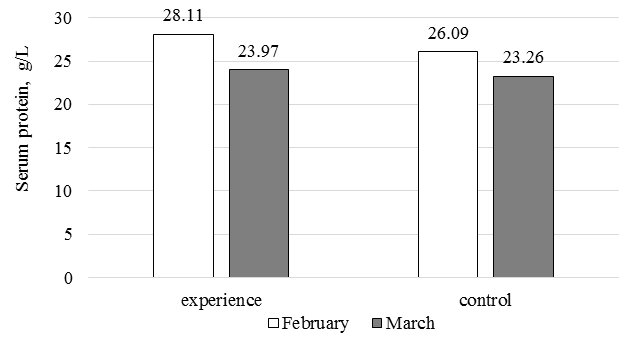
Fig. 3. Dynamics of serum protein in the Russian-Lena sturgeon hybrid
in the experiment on the influence of high concentrations of the E-selenium vitamin-mineral supplement
Hemoglobin levels were sufficient to support these processes, within the range typical for immature sturgeons (55-65 g/L) in natural environments [17]. Erythrocyte sedimentation rate values (3-4.4 mm/hour) were slightly higher than in wild sturgeons [18] in both groups.
The study of cholesterol dynamics also revealed no signs of accelerated generative metabolism [1]. High E-selenium concentrations did not intensify cholesterol synthesis, as evidenced by a 1.7-fold decrease by the experiment's end, reaching levels similar to the control group, where they remained unchanged (Fig. 4).

Fig. 4. Dynamics of cholesterol in the Russian-Lena sturgeon hybrid
in the experiment on the influence of high concentrations of the E-selenium vitamin-mineral supplement
Lipid dynamics in the blood of both groups followed similar trends: a 54% decrease in the experimental group and a 10% decrease in the control. The positive role of E-selenium on blood lipid levels, observed at low concentrations, was not evident at high concentrations. Given the high initial cholesterol levels in the experimental group and its role in cortisol production, the more significant lipid reduction in these fish likely reflects energy expenditure for physiological stabilization and mass gain.
Histological analysis of the gonads confirmed the lack of E-selenium's effect on ovarian development in the Russian-Lena sturgeon hybrid. No signs of accelerated gonad formation were observed in the experimental group. The generative tissue remained at early developmental stages, unchanged from the experiment's start.
Thus, E-selenium, at both low and high concentrations, did not accelerate generative function in the immature Russian-Lena sturgeon hybrid. This was confirmed by metabolic levels and histological analysis of the ovaries. Only a slight trend toward higher mass accumulation in the experimental fish was noted.
The second series of experiments with E-selenium was conducted on mature sterlet × beluga hybrid females.
To evaluate the influence of the E-selenium supplement in sturgeon feed on metabolism, mature sterlet × beluga hybrid females were selected. The average initial mass was 16.68 ± 1.23 kg in the experimental group and 16.20 ± 0.81 kg in the control. By the end, the experimental group's mass increased by 3.0% (17.18 ± 1.07 kg), while the control group's increased by 5.1% (17.02 ± 0.83 kg). Blood parameters (ESR, hemoglobin, serum protein, β-lipoproteins, and cholesterol) were analyzed.
The inclusion of E-selenium in the feed altered nearly all studied blood parameters (Fig. 5), reflecting differences in the physiological state of the experimental and control fish.

Fig. 5. Blood parameters in sterlet × beluga hybrid females: 1 – start of the study, 2 – end of the study
In the control group, ESR increased by 1.5 times by the experiment's end, while it decreased slightly (7%) in the experimental group. Conversely, hemoglobin, serum protein, and β-lipoprotein levels increased in the experimental group compared to the control.
Hemoglobin dynamics, though differing between groups, remained within typical ranges for wild fish (50-80 g/L). By the end, hemoglobin increased by 12.1% in the experimental group (statistically insignificant, p ≥ 0.05), while it decreased by 10% in the control. Total protein levels increased by 35.5% in the experimental group (p ≤ 0.001), with no change in the control. β-lipoprotein levels decreased by 36% in the control but increased by 36% in the experimental group (p ≤ 0.001). Cholesterol levels rose by 63% in the control but decreased by 5% in the experimental group.
Analyzing the dynamics and direction of changes in the studied blood parameters, it is necessary to note their multidirectional nature. Taking into account that the studied fish are sexually mature, and therefore may be at different stages of gonad maturation, the changes in metabolism will correspond to the stages of oocyte maturation [19]. At the beginning of the experiment, ultrasound scans showed that the ratio of fish in different stages of gonadal maturation was the same in both the experimental and control groups: 60% in the second stage, 35% in the third stage, and 5% in the final fourth stage. By the end of the experiment, the number of mature fish (Stage IV of gonadal maturity) in the experimental group had increased sixfold, reaching 30%, while the number of mature fish in the control group was only 15%, which was half as much. The ratio of females in the II and III stages of maturation has also changed. The group of experimental fish had the lowest proportion of immature fish (Stage II of gonadal maturity), which decreased by half compared to the beginning of the experiment, reaching 30%. At the same time, the proportion of fish in the control group that had reached other stages of maturity was 5%, while the proportion of immature fish remained high at 55%. By the end of the experiment, the proportion of fish in the control group with stage III maturity did not change, while the proportion of fish in the experimental group increased by 10%, reaching 40%. This suggests that the addition of E-selenium to the feed accelerates the maturation of females. This is also supported by changes in the metabolism of females in the experimental group. In particular, there is an increase in the blood levels of total protein and β-lipoproteins, which is associated with the vitellogenic stage of oocyte development [19]. Vitellogenesis, a critical oocyte development stage regulated by sex hormones, involves vitellogenin and other egg proteins synthesized by the liver under 17β-estradiol influence, transported to oocytes, and stored as lipovitellin, phosvitin, and other proteins [20, 21]. Vitellogenin shares homology with apolipoprotein B-100 (apoB-100), or β-lipoprotein [22, 23], and is immunochemically identical to β-lipoproteins [24]. In wild sturgeons, β-lipoprotein levels rise during spawning migration and decline as maturation completes (6.4-7.0-2.4 g/L) [25], a pattern also observed in farmed fish [19]. Increased hemoglobin levels support the energy demands of protein and vitellogenin synthesis. Cholesterol also plays an important role in gonadogenesis. The biological role of cholesterol determines the variety of its most important functions, which it performs in the body of any animal, and it also actively participates in the generative metabolism [26]. An analysis of the initial physiological state of female hybrids based on their blood cholesterol levels showed that they were similar in both groups. In wild breeders, during the spawning migration period, the concentration of cholesterol in the blood serum increases by more than two times, from 1.87 to 4.45 mmol/L, from the beginning of active maturation to the end of the process [27]. In different-aged sturgeon fish in the sea, the level of cholesterol rarely exceeds 2.8 mmol/L. In the experimental group, this value did not exceed 3.27-3.10 mmol/L. Increased cholesterol levels during vitellogenesis are usually associated with increased estrogen synthesis, particularly estradiol-17β [28], which stimulates the liver's synthesis of the yolk precursor, vitellogenin [29], and increases generative metabolism.
Thus, the level and direction of changes in the studied blood parameters indicate an intensification of the generative exchange in the female fish of the experimental group.
The analysis of the blood parameters studied in the control batch shows a different picture of changes in the physiological state of the females. It should be noted that these changes are more in line with the physiological state of fish that are in a slightly stressed state [30]. This is indicated by a slight increase in the erythrocyte sedimentation rate, a slight decrease in the hemoglobin content in the blood, and especially an increase in the concentration of cholesterol, which is also the basis for the synthesis of various steroid hormones by the adrenal glands, including cortisol, a stress hormone. At the same time, the intensity of the generative exchange decreases at the end of the experiment, which is indicated by a decrease in β-lipoproteins, which are markers of the intensity of vitellogenin transfer to oocytes.
Thus, E-selenium positively influences the reproductive function of sterlet × beluga hybrid females by stimulating generative metabolism and protecting against oxidative stress, as supported by literature [4-7] and the stressed state of control fish. The synergistic effects of vitamin E and selenium enhance antioxidant and adaptogenic actions, protecting embryos from oxidation, maintaining immunity, and preventing lipid peroxidation in reproductive products.
E-selenium supplementation unlocks the reproductive potential of broodstock, increasing generative productivity.
Conclusion
The use of E-selenium at low or high concentrations in feeds for immature Russian-Lena sturgeon hybrids did not accelerate generative function development. Only a trend toward higher mass accumulation in the experimental fish was observed.
In mature sterlet × beluga hybrids, E-selenium supplementation positively influenced the reproductive system of females, stimulating generative metabolism. Compared to the control, the proportion of mature females (Stage IV of gonadal maturity) doubled, while the proportion of females remaining at stage II maturation decreased by 1.8 times, despite similar initial gonad maturity distributions.
1. Пономарева Е. Н., Гераскин П. П., Металлов Г. Ф. Неваленный А. Н., Григорьев В. А., Сорокина М. Н., Федоровых Ю. В. Особенности изменения функционального состояния осетровых рыб при половом созревании в установках замкнутого водоснабжения // Биология внутренних вод. 2021. № 1. С. 77–84.
2. Zuo Z. H., Shang B. J., Shao Y. C., Li W. Y., Sun J. S. Screening of intestinal probiotics and the effects of feeding probiotics on the growth, immune, digestive enzyme activity and intestinal flora of Litopenaeus vannamei // Fish & Shellfish Immunology. 2019. V. 86. P. 160–168.
3. Beltrán J. M. G., Esteban M. A. Nature-identical compounds as feed additives in aquaculture // Fish & Shellfish Immunology. 2022. V. 123. P. 409–416.
4. Blom J. H. Dabrowski K. Reproductive success of female rainbow trout (Oncorhynchus mykiss) in response to different levels of ascorbyl monophosphate in the diet // Biol. Reprod. 1995. V. 52. P. 1073–1080.
5. Малетич М. Б., Процик Я. М., Ривис И. Ф. Жирнокислотный состав фосфолипидов печени и воспроизводительная способность карпов-производителей при разных уровнях витамина А в комбикорме // Науч. вестн. ЛНУВМБТ им. С. З. Гжицкого. 2015. Т. 17. № 3 (63). С. 241–246.
6. Тимирханова Г. А., Абдуллина Г. М., Кулагина И. Г. Витамин С: классические представления и новые факты о механизмах биологического действия // Вятский мед. вест. 2007. № 4. URL: https://cyberleninka.ru/article/n/vitamin-s-klassicheskie-predstavleniya-i-novye-fakty-o-me-hanizmah-biologicheskogo-deystviya (дата обращения: 19.12.2024).
7. Elgendey F., Wakeel R. A., Hemeda S. A., Elshwash А. М., Fadl S. E., Abdelazim А. М., Alhujaily М., Khalifa О. А. Selenium and/or vitamin E upregulate the antioxidant gene expression and parameters in broilers // BMC Vet Res. 2022. V. 18. P. 310.
8. Tarnecki A. M., Rhody N. R., Walsh C. J. Health characteristics and blood bacterial assemblages of healthy captive red drum: implications for aquaculture and fish health management // Journal of Aquatic Animal Health. 2018. V. 30 (4). P. 339–353.
9. Buchmann K. Control of parasitic diseases in aquaculture // Parasitology. 2022. V. 149 (14). P. 1985–1997.
10. Гречкина О. Ю., Савельев О. А. Использование кормовых добавок в аквакультуре // Актуальные вопросы и перспективы развития сельскохозяйственных наук: сб. науч. тр. по итогам III Междунар. науч.-практ. конф. (Омск, 11 мая 2016 г.). Саратов: Инновац. центр развития образования и науки, 2016. № 3. С. 33–35.
11. Левина О. А. Технологические приемы повыше-ния эффективности товарного осетроводства: автореф. дис. ... канд. с.-х. наук. Усть-Кинельский, 2017. 18 с.
12. Van Kampen E. J., Zijlstra W. G. Standardization of hemoglobinometry. II. The hemoglobincyanide method // Clin. Chim. Acta. 1961. V. 6. Р. 538–545.
13. Fishbach F., Dunning M. A manual of laboratory diagnostic tests. Lppincott Williams & Wilkins, 2004. 1291 p.
14. Лакин Г. Ф. Биометрия. М.: Высш. шк., 1990. 293 с.
15. Шелухин Г. К. Физиолого-биохимическая характеристика осетровых северо-каспийской популяции в морской период жизни // Актуальные вопросы осетрового хозяйства. Астрахань, 1971. С. 214–216.
16. Сергеева Н. Т. О влиянии добавок витамина Е, селена и кальмарового жира в составе комбикорма РГМ-5В на обмен веществ и темп роста форели (Salmo gairdneri Rich.) // Вопросы разработки и качества комбикормов: сб. науч. тр. М.: Изд-во ВНИИПРХ, 1989. Вып. 57. С. 27–31.
17. Лукьяненко В. И., Гераскин П. П. Количественная характеристика гемоглобина крови у осетровых в морской и речной периоды жизни // Тез. отчетной сес. ЦНИОРХ (Астрахань, 22–25 февраля 1966 г.). Астрахань, 1966. С. 41–42.
18. Лукьяненко В. И., Гапонов В. С. Видовые особенности и половой диморфизм РОЭ у осетровых // Материалы науч. сес. ЦНИОРХ (Баку, 18–19 марта 1968 г.). Баку, 1968. С. 54–55.
19. Гераскин П. П., Металлов Г. Ф., Григорьев В. А., Яицкая М. В. Физиолого-биохимические закономерности созревания самок осетровых рыб // Аквакультура: мировой опыт и российские разработки: материалы Всерос. науч. конф. Ростов н/Д.: Изд-во ЮНЦ РАН, 2017. С. 493–496.
20. Lubzens E., Young G., Bobe J., Cerda J. Oogenesis in bony fish: how fish eggs are formed // General and Comparative Endocrinology. 2010. V. 165 (3). P. 367–389.
21. Jena B., Mohanty J., Das R. K., Garnayak S. K., Nandi S. Induction, purification and partial characterization of vitellogenin in the large Indian carp Catla catla (Ham.) // Aquaculture Studies. 2013. V. 44 (12). P. 190–191.
22. Bieberstein U., Berbner T., Islinger M., Braunbeck T. Immunohistochemical localization of vitellogenin in rainbow trout (Oncorhynchus mykiss) hepatocytes using immunofluorescence // Sci. Total Environ. 1999. V. 233. P. 67–75.
23. Baker M. E. Is vitellogenin an ancestor of apolipoprotein B-100 of human low-density lipoprotein and human lipoprotein lipase? // Biochem J. 1988. V. 255 (3). P. 1057–1060.
24. Ипатов В. В., Лукьяненко В. И. Сывороточные белки рыб: гетерогенность, структура и функции // Успехи современной биологии. 1979. Т. 88. Вып. 14. С. 104–124.
25. Субботкин М. Ф. Концентрация сывороточных бета-липопротеидов у каспийских осетровых в морской и речной периоды жизни // Осетровое хозяйство внутренних водоемов СССР. Астрахань, 1979. 253 с.
26. Кнорре Д. Г., Мызина С. Д. Биологическая химия. М.: Высш. шк., 2000. 476 с.
27. Лукьяненко В. И., Шелухин Г. К. Особенности функционального состояния осетровых в морской и речной периоды жизни // Биологические процессы в морских и континентальных водоемах: тез. докл. II съезда ВГБО. Кишинев, 1970. С. 227.
28. Zhao Z., Zhao Q., Wang H., Wei L., Wang S., Li S., Yuan D., Wang Z. Integrated transcriptomic and metabolomic analyses identify key factors in the vitellogenesis of juvenile Sichuan bream (Sinibrama taeniatus) // Front. Mar. Sci. 2023. V. 10. DOI:https://doi.org/10.3389/fmars.2023.1243767.
29. Sun B., Pankhurst N. W. Correlation between oocyte development and plasma levels of steroids and vitellogenin in greenback flounder Rhombosolea tapirine // Abstr. 7th Intern. Symp. on Reproductive Physiology of Fish. 2003. P. 95.
30. Гераскин П. П. Реакции организма каспийских осетровых (Acipenseridae) на загрязнение среды обитания: автореф. дис. … д-ра биол. наук. М., 2013. 35 с.
















