Россия
Россия
Россия
Россия
Россия
Россия
УДК 639.371.7 Разведение сомовых
Африканский клариевый сом Clarias gariepinus (Burchell, 1822) является одним из перспективных объектов выращивания в пресноводной товарной аквакультуре России. На сегодняшний день в перечне Министерства сельского хозяйства Российской Федерации зарегистрировано два селекционных достижения: порода африканского сома «Михайловская» и породная группа «Таманская», обладающие высокими продукционными и адаптивными характеристиками. Рассматривается возможность повышения эффективности производства рыбопродукции из африканского сома посредством использования гибридных групп сомов, полученных путем скрещивания «михайловских» и «таманских» особей. По результатам анализа рыбоводно-биологических, морфометрических и продукционных характеристик было выявлено превосходство гибридного потомства первого поколения по некоторым показателям относительно данных исходных родительских форм. Скрещивание между двумя линиями показало увеличение средней скорости роста длины и абсолютного прироста массы помесей F1 по сравнению со средним показателем родителей (p < 0,05). В анализе главных компонент морфометрических промеров и индексов первая компонента (PC1) отвечала за 25,6 % от общего распределения данных, вторая компонента (PC2) за 16,3 % вариаций формы среди выборок, что указывает на явление гетерозиса в полученной группе из-за высокой вариабельности морфотипов у потомства. По результатам морфометрического анализа было выявлено превосходство гибридов первого поколения по показателям длины спинного и анального плавников (на 15,6 и 9,3 %) по сравнению с «михайловской» породой (p < 0,05). Согласно продукционным индексам гибриды F1 более привлекательны для переработки за счет меньшей длины головы, большей длины, высоты и объема туловища.
гибридное потомство первого поколения, африканский сом, морфометрические показатели, продуктивность, индексы, F1
Introduction
The African catfish Clarias gariepinus (Burchell, 1822) has been successfully farmed in Russia since the early 2000s, but has become more widespread since 2010 and is considered a profitable business. Its natural habitat is lakes, reservoirs, and rivers in sub-Saharan Africa, South America, Southeast Asia, and Europe [1, 2]. Aquaculture farming of clarias catfish is practiced in more than 55 countries worldwide [3]. This species gained wide popularity thanks to the highly productive line bred in Belgium and the Netherlands, known as “Dutch Clarias”, and subsequently transferred to farms in the Central African Republic, South Africa, Côte d'Ivoire, and Nigeria [4-6]. African catfish have a wide range of beneficial biological properties, including rapid growth rate, disease resistance, and tolerance of high stocking density [7]. The fishery development of African catfish in Russia began in the 90s of the 20th centuries through the import of fish from Netherland, and since 2017 the Mikhailovskaya breed (patent No. 9064) was obtained, and since 2019 the Tamanskaya breed group (patent No. 10639).
Hybridization of African catfish is one of the most effective methods for creating new lines of fish with improved characteristics in world practice. In 1988 it was possible to cross C. gariepinus with a local catfish species C. microstomus in Thailand and obtain a hybrid that combines the positive qualities of both parents, such as increased resistance to diseases and faster growth [8]. Similar beneficial properties are demonstrated by other interspecific hybrids: Clarias batrachus and C. gariepinus [9]; C. macrocephalus and C. gariepinus [10]; C. fuscus and C. gariepinus [11]. Crossing lines of African catfish belonging to different ecological zones can have a significant impact on the hybrid offspring. Such interspecific and interline crossing can improve the reproductive qualities of the offspring due to an increase in heterozygosity, which prevents the manifestation of unfavorable recessive traits. The principles of selection in aquaculture are based on the analysis of the main phenotypic and genetic variations of the objects of study, including taking into account the fish-farming and biological parameters of growth and development of aquatic organisms [12, 13]. Hybrids, due to the phenomenon of heterosis, can exhibit various advantages, such as higher growth rates, improved fertility, increased viability and resistance to diseases.
Long-term breeding using the same type of African catfish strains can lead to a loss of genetic variability [14]. Heterozygosity decreases in the offspring obtained as a result of crossing between related individuals, which leads to a decrease in growth performance, fertility and viability due to inbreeding depression [15, 16]. Despite the potential advantages of interline hybridization, there are also negative effects – a decrease in the quality of sexual products, low reproductive capacity, sterility [17]. In particular, this concerns the introduction of genetic material from different populations, leading to a decrease in hybrid growth strength or disease resistance if the individuals used as producers do not have the necessary adaptive qualities for a particular region of cultivation. For example, in African catfish strains in Thailand, due to frequent crossing of wild and domesticated populations, outbreeding depression was found in the resulting offspring, leading to losses in production efficiency [18]. This highlights the importance of careful selection of parental forms to achieve desired results in aquaculture.
The aim of this study is a comparative analysis of the fish-biological, morphometric and production parameters of first-generation African catfish hybrids and the original parental forms of the Mikhailovskaya and Tamanskaya breeds.
Materials and methods
African catfish Clarias gariepinus (Burchell, 1822) of the Mikhailovskaya breed (patent No. 9064), Tamanskaya breed group (patent No. 10639) and their hybrid offspring (♀ Mikhailovskaya × ♂ Tamanskaya) of the first (F1) generation were kept on the basis of the infrastructural resources of the unique scientific installation (USI) of the NTI of the Russian Federation Reg. No. 3662433 – “Research Complex of Advanced Technologies in Aquaculture and Hydroecology” of the Faculty of Biotechnology and Fisheries, Moscow State University of Technologies and Management named after K. G. Razumovsky (FCU). The fish were kept in RAS tanks with a volume of 3 000 l at average stocking densities (30 kg/m3). In the RAS tanks, hydrochemical parameters (t °, O2, pH, NO2, NO3, NH4, PO4) are monitored daily according to ER F. 14.1: 2: 4.112-97; ER 52.24.394-95; ER F 14.1: 2: 4.4-95; ER F 14.1: 2: 43-95. Fish were fed twice a day with specialized feed for catfish according to their age and weight characteristics (extruded production feed for catfish 4-6 mm (Russia)).
Fish farming and biological studies were conducted on four groups of catfish at the initial stage of the study: parental individuals of the Mikhailovskaya breed (weight 388.5 ± 7.7 g, length 32.3 ± 0.6 cm,
n = 15) (Mikhailovskaya group); Tamanskaya (weight 381.1 ± 8.1 g, length 31.3 ± 1.5 cm, n = 15) (Tamanskaya group) and F1 hybrids (weight 394.8 ± 4.7 g, length 31.7 ± 1.5 cm, n = 15) (F1 Hybrid group). Forty-five individuals were taken for the study. Fish farming and biological parameters were measured using generally accepted methods [19, 20]. The absolute and relative weight and length gain, absolute, relative and specific weight and length growth rate, mass accumulation coefficient were studied and the feed coefficient and survival rate were calculated. To ensure statistical accuracy, the fish farming and biological data were presented for the period of growing all fish groups lasting 1 month with a weight of 370 to 400 g with a feeding rate of 2.7%.
Morphological studies were conducted on sexually mature individuals: parental individuals of the Mikhailovskaya breed (weight 1 520 ± 370 g, length 44.5 ± 5.7 cm, n = 15) (Mikhailovskaya group); Tamanskaya (weight 3 950 ± 302 g, length 57.3 ± 4.3 cm, n = 15) (Tamanskaya group) and F1 hybrids (weight 2 040.4 ± 234.9 g, length 53.3 ± 4.9 cm, n = 25) (F1 Hybrid group). A total of 55 individuals were taken for the study. Morphotype analysis was performed according to the methodology [21]. A total of 20 exterior parameters were measured: standard length (SL); antespectral distance (PPD); head length (HL); ante-dorsal distance (PDD); anteventral distance (PVD); anteanal distance (PAD); dorsal fin base length (DFL); pectoral fin length (PFL); anal fin base length (AFL); body depth (BDA); tail base height CPD; body girth (O); dorsal-caudal fin distance (DDCF); snout length (SNL); interorbital distance (ID); eye diameter (ED); head width (HW); distance to the occipital notch (DSO); length of the occipital notch (OFL); width of the occipital notch (OFW).
The production parameters of the experimental groups were assessed using the following indices: elongation (the ratio of the fish body length to its height) (SL/BDA); high back (the ratio of the dorsal fin length to the body length) (DFL/SL); compactness (the ratio of the body girth to the length of the fish) (O/SL); large head (the ratio of the head length to the body length of the fish) (HL/SL), according to the methodology [19].
Comparative data are presented as mean ± standard deviation. Statistical significance was determined using the nonparametric Kruskal–Wallis test according to the distribution of data assessed using the Shapiro–Wilk test. P value < 0.05 was considered statistically significant. Statistical analysis was performed using GraphPad Prism software version 9.0 (GraphPad, San Diego, CA, USA). Principal component analysis (PCA) was performed on the morphometric data set and indices using the R (v3.5.2)/RStudio software package [22] and the factoextra v 1.0.7 package [23]. For the purpose of data normalization, the features characteristic of the trunk region (PPD, PDD, PVD, PAD, DFL, PFL, AFL, BDA, CPD, DDCF) were converted into percentages of the standard body length (SL), and the features characteristic of the head region (SNL, ID, ED, HW, DSO, OFL, OFW) were converted into percentages of the head length (HL).
Results
Growth performance. After a month of cultivation in RAS conditions, the first-generation hybrids reached a final weight of at least 620 g, which significantly exceeded the weight of Mikhailovsky individuals by 12.4% and Tamansky individuals by 27.6% (p < 0.05) (Table 1).
Table 1
Growth performance of parental and hybrid groups of C. gariepinus during a 1-month rearing period*
|
Parameters |
Experienced groups |
||
|
Mikhailovskaya (n = 15) |
Tamanskaya (n = 15) |
F1 hybrid (n = 15) |
|
|
Initial weight, g |
388.5 ± 7.7 |
381.1 ± 8.1 |
394.8 ± 4.7 |
|
Initial length, cm |
32.3 ± 0.6 |
31.3 ± 1.5 |
31.7 ± 0.6 |
|
Final weight, g |
575.6 ± 23.5b |
507.1 ± 71.0b |
647.3 ± 24.1a |
|
Final length, cm |
38.5 ± 1.3 |
38.0 ± 1.0 |
40.3 ± 0.6 |
|
Initial biomass, kg |
5.8 |
5.7 |
6.0 |
|
Final biomass, kg |
8.9 |
7.8 |
9.7 |
|
Body weight gain, g |
187.1 ± 27.9b |
126.0 ± 49.8b |
252.5 ± 25.0a |
|
Absolute increase in length, cm |
6.2 ± 0.8 |
6.7 ± 1.2 |
8.7 ± 0.6 |
|
Absolute rate of weight gain, g/day |
6.45 ± 0.9 |
4.35 ± 1.7 |
8.71 ± 0.8 |
|
Absolute rate of length gain, cm/day |
0.21 ± 0.03 |
0.12 ± 0.04 |
0.3 ± 0.02 |
|
Relative weight gain, % |
48.2 ± 7.8 |
34.2 ± 16.8 |
65.1 ± 14.9 |
|
Relative increase in length, % |
19.0 ± 2.0b |
21.4 ± 4.6ab |
27.3 ± 2.1a |
|
Relative rate of weight gain, %/day |
1.34 ± 0.18 |
0.99 ± 0.41 |
1.68 ± 0.28 |
|
Relative rate of growth of length, %/day |
0.59 ± 0.05 |
0.66 ± 0.12 |
0.83 ± 0.05 |
|
Specific growth rate (weight), % |
1.35 ± 0.18 |
1.0 ± 0.42 |
1.72 ± 0.31 |
|
Specific growth rate (length), % |
0.6 ± 0.05b |
0.67 ± 0.13b |
0.83 ± 0.06a |
|
Mass accumulation coefficient |
0.11 ± 0.01 |
0.05 ± 0.06 |
0.14 ± 0.02 |
|
Food conversion ratio, un. |
1.2 |
1.17 |
1.4 |
|
Survival, % |
92.0 |
92.0 |
94.0 |
* Note here and further: the p-value was calculated on the data set using the nonparametric Kruskal–Wallis test. Superscript letters (a, b, c) indicate statistical significance between group differences at p < 0.05.
The absolute weight gain in the F1 hybrid group was also significantly higher compared to Mikhailovsky by 1.35 times and Tamansky by 2 times (p < 0.05).
Based on the results of the analysis of growth performance, it can be noted that the studied data in the group of first-generation hybrids are comparable with the parent group of the Mikhailovskaya breed in terms of absolute (6.45 to 8.71 g/day), relative (1.34 to 1.68%/day) and specific weight growth rate (1.35 to 1.72%), as well as relative weight gain (48.2 and 65.1%, respectively).
Morphometric analysis. Comparative analysis of morphometric measurements expressed as a percentage of the body and head length of fish are presented in Table 2).
Results of comparative analysis of relative data of morphometric measurements of hybrid groups and parental individuals of C. gariepinus
|
Index |
Experienced groups |
||
|
Mikhailovskaya (n = 15) |
Tamanskaya (n = 15) |
F1 hybrid (n = 25) |
|
|
SL, cm |
44.6 ± 5.68 |
57.3 ± 4.33 |
53.3 ± 4.9 |
|
PDD, % SL |
30.3 ± 2.92 |
28.3 ± 7.29 |
33.5 ± 2.05 |
|
PAD, % SL |
46.5 ± 5.4 |
49.8 ± 2.7 |
53.2 ± 4.91 |
|
PVD, % SL |
40.4 ± 3,24b |
43.9 ± 1.65ab |
46.7 ± 2.79a |
|
PPD, % SL |
25.6 ± 2.4 |
21.3 ± 0.96 |
23.5 ± 4.74 |
|
DFL, % SL |
55.2 ± 3.89b |
61.1 ± 1.77ab |
63.8 ± 3.74a |
|
AFL, % SL |
39.4 ± 8.62b |
42.1 ± 1.04ab |
43.1 ± 1.77a |
|
PFL, % SL |
11.5 ± 1.27 |
12.3 ± 0.95 |
11.7 ± 1.28 |
|
DDCF, % SL |
4.9 ± 0.59 |
7.1 ± 2.25 |
4.3 ± 0.94 |
|
CPD, % SL |
9.5 ± 0.59 |
8.6 ± 0.47 |
9.0 ± 0.99 |
|
O, % SL |
40.3 ± 3.23 |
37.3 ± 2.73 |
40.2 ± 5.79 |
|
BDA, % SL |
18.3 ± 1.46 |
17.2 ± 1.34 |
18.0 ± 1.97 |
|
HL, % SL |
23.4 ± 1.6 |
24.4 ± 2.73 |
24.5 ± 3.74 |
|
HW, % HL |
13.9 ± 0.91b |
18.2 ± 1.45ab |
18.9 ± 1.11a |
|
SNL, % HL |
31.0 ± 2.87a |
24.3 ± 2.8b |
29.5 ± 4.84a |
|
ID, % HL |
42.5 ± 3.38 |
48.3 ± 3.94 |
48.6 ± 6.47 |
|
ED, % HL |
6.1 ± 0.59 |
6.5 ± 0.92 |
4.5 ± 1.89 |
|
OFL, % HL |
11.6 ± 2.96 |
12.3 ± 0.33 |
14.9 ± 3.64 |
|
OFW, % HL |
4.9 ± 0.18b |
4.7 ± 0.52b |
7.0 ± 2.17a |
|
DSO, % HL |
59.5 ± 7.33b |
62.4 ± 9.12ab |
83.5 ± 13.41a |
According to statistical analysis, the first-generation hybrids had a significant advantage over the Mikhailovskaya group in the following parameters of the trunk region: PVD (anteventral distance) and DFL (dorsal fin length) are 1.15 times greater (by 15.6 and 13.4%), AFL (anal fin length) is 1.09 times greater (by 9.3%). Head section: HW (head width) is 1.35 times greater than Mikhailovskaya (by 36%), SNL (snout length) is 1.2 times greater than Tamanskaya (by 21.3%), OFW (occipital notch width) is 1.4 and 1.5 times greater than Mikhailovskaya (by 30%) and Tamanskaya (by 23%) breed groups, respectively, DSO (distance to the occipital notch) is 1.4 times greater than Mikhailovskaya (by 28.7%).
However, the parental breed groups also had significant differences from the hybrids in the following parameters: the Tamanskaya group had a greater length of the distance between the dorsal and caudal fins (DDCF) by 1.65 times (by 39.4%) compared to the F1 hybrids. Significant differences were also found in the eye diameter (ED). ED in F1 hybrids was less than Mikhailovskaya and Tamanskaya by 1.35 and
1.4 times (by 35.5 and 44.4%). Some morphometric measurements did not have statistically significant differences between the groups CPD (tail base height) and ID (interorbital distance).
The F1 specimen's measurements are superior to the parent lines in all respects: a longer and wider body, an elongated head, and also have significant differences in the length and width of the occipital cavity.
In turn, based on the results of the principal component analysis, three clusters are identified on the graph of projections of the original and hybrid forms of the clariid catfish, the first of which is formed by the parental individuals of the Mikhailovskaya breed and is shifted to the lower right part of the graph relative to the component (PC1), the second cluster is formed by the parental group of Tamanskaya individuals and occupies the upper right part of the graph (Fig. 1). Data separation regarding the second component (PC2) was minimal.

Fig. 1. Projection graph of observations of principal component analysis of morphometric measurements
and indices of parental and first-generation hybrid group Clarias gariepinus: PC1 – the first principal component; PC2 – the second principal component
At the same time, hybrid individuals formed a group occupying the central and left sides of the graph, partially intersecting with Tamanskaya individuals in the central part. Some hybrid specimens demonstrated significant differences, but such variability in F1 hybrids can be explained by a high degree of heterosis.
The correlation graph of variables (Fig. 2) shows that from the entire set of considered features, such indicators as SL/BDA (length of the body), ID (interorbital distance), DSO (distance to the occipital fossa), OFL (length of the occipital fossa), O/SL (compactness), DFL/SL (high back), HL/SL (large head), PVD (anteventral distance), HW (head width), HL (head length), OFL (length of the occipital fossa) make a significant contribution to the distribution of data according to the principal component method.
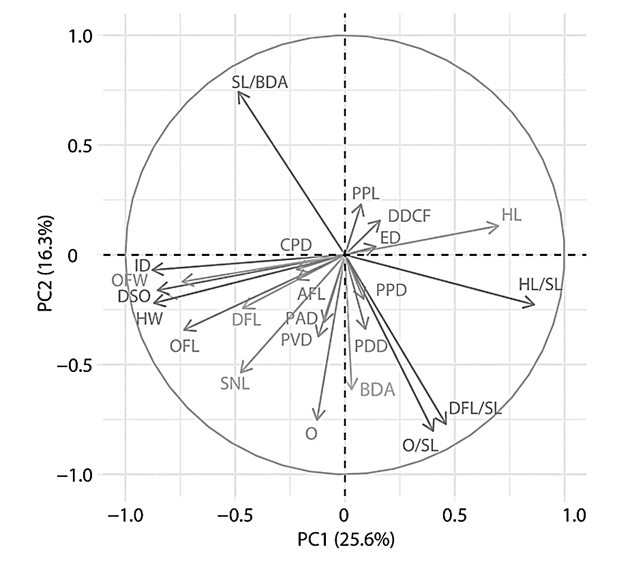
Fig. 2. Correlation graph of variables of principal component analysis of exterior indicators
and indices of studied groups of Clarias gariepinus
On the contrary, the influence of such features as PFL (pectoral fin length), DDCF (distance between the dorsal and caudal fins), PPD (antespectral distance), AFL (length of the anal fin base), CPD, and ED (eye diameter) on the distribution of data is minimal.
Productivity indices. According to calculations, the main indices of productivity of individuals, such as elongation (SL/BDA), are characteristic of the Mikhailovskaya (2.5%) and Tamanskaya (3.2%) breeds and F1 hybrids (3.0%), compactness (O/SL) and large-headedness (HL/SL) of the Mikhailovskaya breed (0.9 and 0.53%) (Table 3).
Table 3
Results of comparative analysis of morphometric indices of first-generation hybrids and parental individuals of C. gariepinus
|
Index |
Experienced groups |
||
|
Mikhailovskaya (n = 15) |
Tamanskaya (n = 15) |
F1 hybrid (n = 25) |
|
|
Elongation (SL/BDA) |
2.5 ± 0.49 |
3.2 ± 0.46 |
3.0 ± 0.43 |
|
High-spine (DFL/SL) |
1.3 ± 0.19b |
1.2 ± 0.12ab |
1.1 ± 0.06a |
|
Compactness (O/SL) |
0.9 ± 0.19b |
0.8 ± 0.12b |
0.7 ± 0.09a |
|
Big-headedness (HL/SL) |
0.53 ± 0.07b |
0.46 ± 0.11b |
0.42 ± 0.07a |
The results of the index analysis revealed that hybrid individuals have a reliable difference in the larger direction relative to the parental forms of the Mikhailovskaya and Tamanskaya breeds in terms of compactness (1.28 and 1.14 times) and large-headedness (1.26 and 1.09 times) (p < 0.05). However, a statistically reliable difference in the high-spine index (1.18 times) was revealed only between hybrids and the Mikhailovskaya breed group.
Thus, the effectiveness of selection based on morphometric indicators of productivity is traced by the indices: first generation hybrids are characterized by a smaller head and a higher and shorter body, at the stage of primary processing with a high yield of commercial products.
Discussion
Based on the conducted research, the obtained intercross hybrid of the first-generation African catfish (♀ Mikhailovskaya × ♂ Tamanskaya) has the desired useful qualities – a comparatively higher growth rate of length, a smaller head, greater length and girth of the body. Growth performance indicators (absolute, specific, relative growth rate) in the future can be improved through a more intense selection of producers according to these indicators and the selection of highly nutritious feed. The exterior parameters of catfish show that the parental groups of the Mikhailovskaya breed and the Tamanskaya breed group are isolated by morphotype, first-generation hybrids demonstrate an insignificant spread, while forming a separate cluster formation. Thus, further selection by morphometric characteristics will allow selecting a homogeneous group with high prognostic criteria of productivity.
A frequent phenomenon in fish farming complexes is a decrease in the productivity and viability of the objects of cultivation. Genetic diversity plays a critical role in the productivity of the offspring in the hybridization of African catfish. It contributes to an increase in the adaptive potential and resistance of the offspring to various stress factors. This is due to the fact that hybrids with greater geno- and phenotypic variance more often demonstrate the phenomenon of heterosis (hybrid vigor), which is manifested in faster growth, better feed conversion and increased fertility [24]. Inbreeding is inevitable in the lines used by industrial farms due to crossing between genetically related individuals [16]. An increase in homozygosity leads to a loss of fitness, since heterozygous advantages are reduced and recessive genes dominate. Given the negative experience of cultivating introduced North African catfish with low genetic diversity in fish farms in Thailand [14], it is proposed to introduce a system of selecting parental individuals and conducting interline crossings in African catfish lines in domestic aquaculture farms, as recommended in the work with Spanish killifish [25]. A positive effect is demonstrated by crossings between genetically differentiated and inbred populations of Penaeus stylirostris, leading to improved productivity due to the effect of heterosis and restoration of F1 from inbreeding [26]. As shown by the results of hybridization in Poecilia reticulata populations, a similar approach that promotes the emergence of heterosis can be the use of genetically different lines (large genetic distance between lines, measured using neutral molecular markers) [27].
Conclusion
First-generation hybrids of Clarias gariepinus (Mikhailovskaya × Tamanskaya) are characterized by a comparatively greater final weight (by 21 and 12%) and absolute weight gain (by 26 and 50%) compared to the parent groups due to the assumed heterosis effect.
F1 hybrids have a statistically significantly greater body length (DFL and AFL) by 15 and 9% compared to the Mikhailovskaya breed.
According to production indices, F1 hybrids are more attractive for technological processing due to the shorter head length, greater length, height and volume of the body.
In the context of breeding work with African catfish, it is recommended to use interline crossing to obtain first-generation offspring demonstrating phenotypic superiority over the parent individuals.
1. Sanda M. K., Metcalfe N. B., Capstick M., Nichols J., Mable B. K. Genetic diversity, population structure and differentiation of farmed and wild African catfish (Clarias gariepinus) in Nigeria // bioRxiv. 2024. P. 2024.10. 15.618429.
2. Truter M., Hadfield K. A., Smit N. J. Review of the metazoan parasites of the economically and ecologically important African sharptooth catfish Clarias gariepinus in Africa: Current status and novel records // Advances in Parasitology. 2023. V. 119. P. 65–222.
3. Chandra Segaran T., Azra M. N., Piah R. M., Lananan F., Tellez-Isaias G., Gao H., Torsabo D., Kari Z. A., Noordin N. M. Catfishes: A global review of the literature // Heliyon. 2023. V. 9. N. 9. P. e20081.
4. Holčík J. Fish introductions in Europe with particular reference to its central and eastern part // Canadian Journal of Fisheries and Aquatic Sciences. 1991. V. 48. N. S1. P. 13–23.
5. Huisman E. A., Richter C. J. J. Reproduction, growth, health control and aquacultural potential of the African catfish, Clarias gariepinus (Burchell 1822) // Aquaculture. 1987. V. 63. N. 1–4. P. 1–14.
6. Roodt-Wilding R., Swart B. L., Impson N. D. Genet-ically distinct Dutch-domesticated Clarias gariepinus used in aquaculture in southern Africa // African Journal of Aquatic Science. 2010. V. 35. N. 3. P. 241–249.
7. Климук А. А., Бекетов С. В., Калита Т. Л. Физиолого-экологические особенности выращивания африканского клариевого сома Clarias gariepinus // Успехи современной биологии. 2024. Т. 144. № 6. С. 705–16. DOI:https://doi.org/10.31857/S0042132424060075.
8. Abit L. Y., Mojilis M. I., Latif K. Successful hybridization between Clarias microstomus♂ and Clarias gariepinus♀ // Aquaculture, Aquarium, Conservation & Legislation. 2023. V. 16. N. 6. P. 3285–3295.
9. Rahman M. A., Bhadra A., Begum N., Islam M. S., Hussain M. G. Production of hybrid vigor through cross breeding between Clarias batrachus Lin. and Clarias gariepinus Bur // Aquaculture. 1995. V. 138. N. 1-4. P. 125–130.
10. Dedukh D., Lisachov A., Panthum T., Singchat W., Matsuda Y., Imai Y., Janko K., Srikulnath K. Meiotic deviations and endoreplication lead to diploid oocytes in female hybrids between bighead catfish (Clarias macrocephalus) and North African catfish (Clarias gariepinus) // Frontiers in Cell and Developmental Biology. 2024. V. 12. P. 1465335.
11. Wembiao Z., Jionghua P., Wensheng L. Culture of catfish in China // Aquaculture. 1988. V. 75. N. 1-2. P. 35–44.
12. Власов В. А., Маслова Н. И., Павлов А. Д. Сохранение и восстановление генофонда рыб аквакультуры России // Изв. Тимирязев. с.-х. акад. 2012. № 5. С. 83–92.
13. Таразевич Е. В. Технологические аспекты формирования ремонтно-маточных стад форели, адаптированных к условиям Беларуси: моногр. Минск: Изд-во БГАТУ, 2022. 190 с.
14. Wachirachaikarn A., Rungsin W., Srisapoome P., Na-Nakorn U. Crossing of African catfish, Clarias gariepinus (Burchell, 1822), strains based on strain selection using genetic diversity data // Aquaculture. 2009. V. 290. N. 1-2. P. 53–60.
15. Koolboon U., Koonawootrittriron S., Kamolrat W., Na-Nakorn U. Effects of parental strains and heterosis of the hybrid between Clarias macrocephalus and Clarias gariepinus // Aquaculture. 2014. V. 424. P. 131–139.
16. Tine M., Ndiaye F., Bale K., Magblenou L. D., Sene M. A. Effects of inbreeding depression on the success of artificial reproduction in the African catfish Clarias gariepinus (Burchell, 1822) // Int. J. Aquac. Fish. Sci. 2022. V. 8. P. 045–053.
17. Na-Nakorn U., Rangsin W., Boon–ngam J. Allotriploidy increases sterility in the hybrid between Clarias macrocephalus and Clarias gariepinus // Aquaculture. 2004. V. 237. N. 1-4. P. 73–88.
18. Patta C., Panthum T., Thatukan C., Wongloet W., Chalermwong P., Wattanadilokchatkun P., Thong T., Srikampa P., Singchat W., Ahmad S. F., Noito K., Rasoarahona R., Kraichak E., Muangmai N., Chatchaiphan S., Sriphairoj K., Hatachote S., Chaiyes A., Jantasuriyarat C., Chailertlit V., Suksavate W., Sonongbua J., Prasanpan J., Payungporn S., Han K., Antunes A., Srisapoome P., Koga A., Duengkae P., Matsuda Y., Na-Nakorn U., Srikulnath K. Questioning inbreeding: Could outbreeding affect productivity in the North African catfish in Thailand? // Plos one. 2024. V. 19. N. 5. P. e0302584.
19. Правдин И. Ф. Руководство по изучению рыб (преимущественно пресноводных). М.: Пищ. пром-сть., 1966. 372 c.
20. Купинский С. Б. Продуктивные возможности объектов аквакультуры. Астрахань: Изд-во ДФ ФГОУ ВПО АГТУ. 2007. 133 c.
21. Legendre M., Teugels G. G., Cauty C., Jalabert B. A comparative study on morphology, growth rate and reproduction of Clarias gariepinus (Burchell, 1822), Heterobranchus longifilis Valenciennes, 1840, and their reciprocal hybrids (Pisces, Clariidae) // Journal of Fish Biology. 1992. V. 40. N. 1. P. 59–79.
22. RStudio Team RStudio: integrated development for R. RStudio, PBC, Boston, MA. 2020. URL: https://posit.co/download/rstudio-desktop/ (дата обращения: 01.03.2025).
23. Kassambara A., Mundt F. Extract and visualize the results of multivariate data analyses. R package factoextra version 1.0.7. // Computer Sci. 2020. URL: https://rpkgs.datanovia.com/factoextra/index.html (дата обращения: 01.03.2025).
24. Власов В. А., Маслова Н. И. Гетерозис в рыбоводстве // Изв. Тимирязев. с.-х. акад. 2015. № 4. С. 82–94.
25. Schönhuth S., Luikart G., Doadrio I. Effects of a founder event and supplementary introductions on genetic variation in a captive breeding population of the endangered Spanish killifish // Journal of Fish Biology. 2003. V. 63. N. 6. P. 1538–1551.
26. Goyard E., Goarant C., Ansquer D., Brun P., de Decker S., Dufour R., Galinie C., Peignon J., Pham D., Vourey E., Harache Y., Patrois J. Cross breeding of different domesticated lines as a simple way for genetic improvement in small aquaculture industries: Heterosis and inbreeding effects on growth and survival rates of the Pacific blue shrimp Penaeus (Litopenaeus stylirostris) // Aquaculture. 2008. V. 278. N. 1-4. P. 43–50.
27. Shikano T., Taniguchi N. Relationships between genetic variation measured by microsatellite DNA markers and a fitness-related trait in the guppy (Poecilia reticulata) // Aquaculture. 2002. V. 209. N. 1–4. P. 77–90.
















