Россия
Россия
Рассматривается опыт применения астаксантина в кормлении стерляди и его влияние на физиологическое состояние особей. Использован набор применяемых в рыбохозяйственных исследованиях методов: вариационной статистики и физиолого-биохимических тестов. Исследовано 125 особей стерляди. Полученные в ходе исследования данные свидетельствуют о том, что использование астаксантина в концентрации 20,0; 30,0; 40,0 и 50,0 мг дает наилучшие физиолого-биохимические показатели культивируемых особей в сравнении с контрольным вариантом. Наиболее эффективной и безопасной концентрацией астаксантина в рационе ценных объектов аквакультуры является 40,0 мг/кг корма, что подтверждается и результатами физиологического состояния особей. Показатели энергетического обмена свидетельствуют о лучшем накоплении пластических веществ у рыб экспериментальной группы, употреблявших корма с вводом астаксантина 40 мг/кг, в данном опытном варианте уровень общего белка у рыб был достоверно выше на 26,4 % (р ≤ 0,05) в сравнении с контрольной выборкой, концентрация гемоглобина составила 77,4 г/л, что на 17,6 % выше, чем у рыб контрольной группы. Использование комбикорма с астаксантином оказало существенное влияние на содержание каротиноида и витамина А в печени, в опытных вариантах эти значения были выше в 2,0 раза по сравнению с контрольной выборкой. Накопление каротиноида и витамина А в печени является благоприятным признаком хорошего физиологического состояния объектов аквакультуры. Проведенные исследования в целом показали, что использование натурального астаксантина в составе кормов для осетровых рыб является эффективным, приводит к улучшению физиологического состояния культивируемых особей.
астаксантин, стерлядь, физиолого-биохимические показатели, лейкоцитарная формула, печень, витамин А
Introduction
Currently, the development of aquaculture is one of the important directions of the agro-industrial sector, which allows providing the population with products of aquatic biological resources. Global aquaculture production has shown impressive growth over the past decades. In 2018, the global production of hydrobionts amounted to 179 million tons. Consumption was expressed in the amount of 20.5 kg per capita per year. The share of the aquaculture sector in the global consumption of fish and seafood is 46% of the total volume and 52% for consumption directly for food. Since 2016 aquaculture has become the main source
of fish for human consumption on a global scale. By 2030 its share is projected to be 59% [1].
The increase in the production of aquaculture products confirms the relevance of the development of feed production in order to provide the industry with high-quality compound feeds and feed additives. An important criterion for choosing feed additives is environmental safety. An ideal antioxidant should be easily absorbed by the body and prevent the formation of free radicals at physiologically significant levels. Therefore, the use of natural antioxidants is of particular interest [2].
One of the main stages of metabolism in the body is the digestibility and digestibility of feed nutrients, the effectiveness of which to a certain extent depends on the use of biologically active substances in diets that have antioxidant properties and have a stimulating effect on vital body functions. When developing the composition of recipes for complete dry combined feeds in industrial aquaculture, it is necessary to pay attention to the presence of a number of irreplaceable biologically active feed components in them. Among them, along with vitamins and minerals, are carotenoids – natural pigments contained in the natural food of fish [3].
The role of carotenoids for the course of normal physiological processes is indisputable. Scientists have found out the immunostimulant role of carotenoids. Carotenoids increase cytostatic activity of killer cells, slow down tumor growth and accelerate wound healing [4–6]. Their importance in increasing the body's resistance to exposure to toxic substances under hypoxia conditions is also noted [7, 8]. A large number of carotenoid pigments were found in the tissues and organs of hydrobionts [9].
In this scientific study, astaxanthin of natural origin has been studied, it has antioxidant, provitamin and antimutagenic activity, is used in the food industry, agriculture and medicine [4].
The aim of the research was to study the effect of the antioxidant astaxanthin of natural origin on the physiological state of sterlet.
Materials and methods of research
Experimental work was carried out on the basis of the Innovation Center “Bioaquapark – STC of Aquaculture” of the Astrakhan State Technical University. The objects of the study were yearlings of sterlet (Acipenser ruthenus, Linnaeus, 1758). The scientific work included an assessment of the effectiveness of the use of the natural antioxidant astaxanthin in feeds for valuable fish species.
Astaxanthin is a carotenoid that, compared to beta–carotene, has two additional oxygen atoms on each of the six-membered rings, belonging to the xanthophyll group. The presence of chromophore groups (conjugated double bonds and quinoid groupings in rings) gives astaxanthin a rich red color.
According to the recommendations of S. V. Ponomarev (2010) [4], the introduction of astaxanthin up to 50 mg per 1 kg of feed is allowed for sturgeon fish. The use of astaxanthin in fish feed is approved by the Food and Drug Administration (FDA). Astaxanthin is registered as a food additive E161j.
The bioavailability of astaxanthin is not too high, but the absorption of astaxanthin improves when combined with edible oils, such as fish oil. Astaxanthin is a lipophilic compound, it dissolves well in oils. Before being introduced into experimental feeds, astaxanthin was previously dissolved in liquid fish oil. The study of diets with different levels of fat showed that the fat content of feed for valuable aquaculture objects should be from 4-8% [10].
The study on the cultivation of sterlet yearlings was carried out on five experimental groups. So, the first group (control) received a food product balanced in all nutrition elements, according to physiological needs. The second group (Test 1) received the diet of the 1st group with the addition of the natural antioxidant astaxanthin in the amount of 20 mg/kg. The third group (Test 2) received the diet of the 1st group with the addition of astaxanthin in the amount of 30 mg/kg. The fourth group (Test 3) received the diet of the 1st group with the addition of astaxanthin in the amount of 40 mg/kg. The fifth group (Test 4) received the diet of the 1st group with the addition of astaxanthin in the amount of 50 mg/kg (Table 1).
Table 1
Scheme of the main parameters of the tests in the cultivation of sterlet yearlings (Acipenser ruthenus, Linnaeus, 1758)
|
Indicators |
Tests |
||||
|
Control |
Test 1 |
Test 2 |
Test 3 |
Test 4 |
|
|
Diet |
Basic diet (BD) |
BD + 20 mg/kg astaxanthin |
BD + 30 mg/kg astaxanthin |
BD + 40 mg/kg astaxanthin |
BD + 50 mg/kg astaxanthin |
|
Granule size, mm |
2.0 |
||||
|
Tanks |
Tank ICA-1 |
||||
|
Stocking density, pcs./m2 |
25 |
||||
|
Feeding method |
Manually, by eatability |
||||
|
Research period, days |
40 |
||||
|
Survival rate, % |
100 |
||||
Cultivation was carried out at the same planting density and constant temperature regime in accordance with the biological feature of the species. The growing conditions are shown in Fig. 1.
Fish feeding was carried out manually 2 times in the daytime. The daily feeding rate was determined according to the feed tables depending on the average weight of fish and water temperature [2].
To assess the possibility of using and the effectiveness of the use of a component of carotenoid origin as an antioxidant source, taking into account the biological characteristics of the nutrition of cultivated fish, feeds were developed, including the natural antioxidant astaxanthin in the amount of 20, 30, 40, 50 mg per 1 kg of feed.

Fig. 1. Growing conditions
The control and experimental feeds were made in laboratory conditions using feed components of domestic production by wet pressing, the conditions for the manufacture of all feed variants were the same (Fig. 2).
The physiological state of the studied objects was assessed by biochemical parameters of protein, lipid and carbohydrate metabolism (blood composition), according to the developed methods [11–14].
|
|
|
|
|
|
|
а |
b |
c |
d |
e |
Fig. 2. Samples of experimental feed for sterlet: a – Control; b – Test 1; c – Test 2; d – Test 3; e – Test 4
The level of beta-lipoproteins was determined by the Burstein method [15]. Blood was taken in vivo from the tail vein of fish into Eppendorf tubes (Fig. 3).

Fig. 3. Taking blood from the caudal vein in the studied hydrobionts
A Unico 2100 spectrophotometer was used to measure the optical density of the samples obtained.
The following indicators were determined: the concentration of hemoglobin photometrically using a set of reagents from Agat-Med [16], the rate of erythrocyte sedimentation (ESR) on the device of R. P. Panchenkov [17].
Blood smears were prepared using a dye fixative from Olvex-diagnosticum (Russia) using the May-Grunwald method [17, 18]. Identification of leukocytes by stages of their cytogenesis, evaluated by differential counting of cell types. 200 leukocytes were identified on each blood smear of juvenile sterlet, taking into account their cytogenesis according to the classification of N. T. Ivanova [18].
The leukocyte shift index was calculated by the formula [18]
![]()
The study of the smallest details on histological blood samples was performed with an Olympus electron microscope (Japan).
The content of carotenoid pigments and vitamin A in the liver of reared fish was determined by colorimetric method on a Unico 2100 spectrophotometer [19]. Considering the physiological role of the astaxanthin carotenoid in a living organism, it was advisable to consider the retinol-forming function, since in the study astaxanthin was previously dissolved in fish oil, for better passive diffusion in the intestinal epithelium. Fish oil is standardized according to the content of vitamin A – at least 350 IU/g.
The results of the research were processed using generally accepted methods of biological statistics and the Microsoft Excel program. The level of differences was assessed using the Student's reliability criterion [20].
Research results
Hematological and biochemical indicators of blood are an adequate indicator of the quality and balance of the feed consumed.
One of the elements of the biochemical assessment of the physiological state of cultured fish is the characteristic of the metabolic function of blood [21]. In order to identify changes in metabolic processes, the dynamics of such indicators as ESR, hemoglobin concentration, leukocyte formula, leukocyte shift index of total serum protein, glucose, beta-lipoproteins, albumin and cholesterol in blood serum were studied.
The data of physiological indicators of sturgeon fish in natural conditions were used as normative values: ESR – 2.0-4.0 mm/h, hemoglobin – 50.0-80.0 g/l, cholesterol – 1.0-3.5 mmol/l, total protein – 20.0-40.0 g/l, beta-lipoproteins – 1.0-5.0 g/l, glucose – 3.0-6.5 g/l [22]. The results of the analysis of the physiological state of the sterlet are presented in Table 2.
Table 2
Hematological parameters of sterlet blood* (n = 25)
|
Indicator |
Control |
Test 1 |
Test 2 |
Test 3 |
Test 4 |
|
Hemoglobin, g/l |
62.8 ± 6.5 65.8 ± 4.8 |
54.9 ± 2.9 71.3 ± 3.7** |
55.0 ± 3.3 70.8 ± 4.2** |
56.2 ± 2.01 77.4 ± 4.04** |
58.0 ± 1.4 72.8 ± 1.2** |
|
ESR, mm/h |
2.2 ± 0.6 2.7 ± 0.3 |
1.5 ± 0.3 2.6 ± 0.4 |
1.8 ± 0.3 2.8 ± 0.2 |
2.0 ± 0.8 2.5 ± 0.7 |
2.3 ± 0.4 3.0 ± 0.6 |
* Numerator – the beginning of the experiment, denominator – the end of the experiment; ** p ≤ 0.05 – the differences are significant.
The rate of ESR and hemoglobin level in all variants of the experiment remained within the normative values, which indicates a constant protein composition of blood plasma [22]. According to the ESR indicator, no differences were found in the study variants (p > 0.05).
As can be seen from the results given in accordance with Table 2, there is a positive trend in the use of astaxanthin in experimental variants, which affects the increase in hemoglobin levels. The concentration of hemoglobin in variants 1, 2, 3 and 4 was: 71.3, 70.8, 77.4 and 72.8 g/l, respectively, which is 7.6, 8.4, 17.6 and 10.6% higher than in the fish of the control group. The high hemoglobin content in the experimental groups (within the reference values) may be associated with a more intensive metabolism in the body of fish. Since the antioxidant astaxanthin was used in the feed, which increases the rheological parameters of the blood, which suggests a positive effect on the microcirculation of blood in the body of fish. The blood circulation depends on how well the organs are supplied with nutrients and oxygen. It is difficult to underestimate this property of astaxanthin [4].
A fairly informative indicator in assessing the overall physiological state of the body is the leukocyte blood formula, which reflects not only the physiological state of fish, but also some aspects of cellular immunity. Changes in the leukogram can detect metabolic disorders and deterioration of the condition of the studied object long before the appearance of clinical signs of emerging pathologies [23]. The leukocyte formula of the blood of the fish under study is presented in accordance with Fig. 4.

Fig. 4. The ratio of shaped blood elements in sterlet
The fish was characterized by a lymphocytic profile – the basis of white blood was lymphocytes, the proportion of which in the total pool of leukocytes does not fall below 70% and did not exceed 90%. Lymphocytes are responsible for immune surveillance, formation and regulation of cellular and humoral immune response, synthesizing protective antibodies, lysing foreign cells, ensuring the destruction of their own mutant cells.
As can be seen from Fig. 4, the proportion of lymphocytes in the studied experimental groups ranged from 74.7-76.0% (p > 0.05) these indicators correspond to the physiological norm for fish. The indices of leukocyte shift in fish in the experimental versions of the study did not differ significantly, the indicators ranged from 0.26-0.28%, these indicators were within the reference values, for sturgeon fish these values range from 0.25-0.40% [24].
The dynamics of biochemical indicators serves as a marker of the state of the fish organism in artificial and natural reservoirs, characterizes the quality and quantity of nutrition, planting density, adaptive abilities of fish, the intensity of anthropogenic factors [25]. The results of the biochemical study are presented in Table 3.
Table 3
Physiological and biochemical parameters of sterlet blood* (n = 25)
|
Indicator |
Control |
Test 1 |
Test 2 |
Test 3 |
Test 4 |
|
Total protein, g/l |
28.5 ± 1.5 28.8 ± 9.5 |
21.6 ± 1.9 35.2 ± 1.6** |
24.6 ± 2.6 33.8 ± 8.7** |
23.1 ± 3.7 36.4 ± 9.3** |
22.3 ± 2.2 34.0 ± 1.8** |
|
Glucose, g/l |
2.2 ± 0.2 2.5 ± 0.8 |
2.6 ± 0.7 3.7 ± 0.6** |
2.5 ± 0.2 3.6 ± 0.5** |
2.5 ± 0.2 4.0 ± 1.2** |
2.3 ± 0.1 3.5 ± 0.7** |
|
Cholesterol, mmol/l |
3.3 ± 0.2 3.5 ± 0.2** |
3.5 ± 0.3 2.4 ± 0.2 |
3.4 ± 0.2 2.5 ± 0.3 |
3.0 ± 0.4 2.0 ± 0.1 |
3.2 ± 0.6 2.6 ± 0.2 |
|
β-lipoproteins, g/l |
3.3 ± 0.2 3.5 ± 0.3 |
3.0 ± 0.3 3.3 ± 0.2 |
2.8 ± 0.4 3.5 ± 0.2 |
2.9 ± 0.5 3.7 ± 0.4 |
2.7 ± 0.1 3.4 ± 0.5 |
|
Albumin, g/l |
24.9 ± 5.1 31.6 ± 0.3 |
22.0 ± 4.3 32.0 ± 1.2 |
25.8 ± 0.4 33.0 ± 1.2 |
27.0 ± 1.8 34.0 ± 1.5 |
25.0 ± 0.9 32.0 ± 1.1 |
* Numerator – the beginning of the experiment, denominator – the end of the experiment; ** p < 0.05 – the differences are significant.
The level of total whey protein at the beginning of the study for Tests 1–4 was low, but was within the normative values and was relatively homogeneous. The variability was 1.5-4.0%. The obtained low data on the concentration of total protein in the blood before the start of the experiment, most likely, indicate a high level of stress load, which is overcome due to the additional energy obtained during protein breakdown. At the end of the experiment, the indicators of total protein were significantly higher in the experimental variants (feed with the addition of astaxanthin), by 17.4, 18.1, 22.2 and 26.4% in comparison with the control sample, the best indicators were characteristic of Test 3.
At the end of the experiment, the glucose concentration in the experimental tests was within the physiological norm (from 3.0-4.0 g/l), which is the result of the normal operation of the enzymatic system that catalyzes the transformation of glucose, the control group was characterized by low concentration values glucose levels are below the reference values [22]. The experimental variants of the study were characterized by data that significantly differed in contrast to the control sample (p < 0.05).
For the growth of the body and cell division, the concentration of cholesterol in the blood plays an important role, which comes from food or is produced by its own cells and synthesized in the liver [21]. The cholesterol level in the blood of fish above 3.7 g/l is considered pathological and indicates the effect of stressful environmental factors [22]. In the conditions of the study, there was an unambiguous tendency to decrease cholesterol in the blood of fish that consumed feed with the addition of astaxanthin to the feed formulation. At the same time, the greatest changes occurred in Test 3 (a decrease in this indicator by 75.0%), while in Tests 1, 2 and 4 it decreased by 20.0, 25.0 and 23.1%, respectively. In the control Test, the cholesterol level was significantly higher (p < 0.05) and did not even change slightly in comparison with the initial data.
The most cholesterol-rich particles are β-lipoproteins (low-density cholesterol) [21]. Under growing conditions, this indicator was at the level of 2.8-3.05 g/l, which corresponds to the values (1.0-5.0 g/l) characteristic of fish from natural habitats [22]. There were no differences in the level of beta-lipoprotein concentration (p > 0.05).
The term “total protein” refers to the total concentration of albumin and globulin in the blood serum. Of all the proteins, albumin synthesized in the liver is present in the highest concentration in plasma. It is necessary to maintain osmotic balance, ensuring the normal distribution of fluid between the blood vessels and the extravascular space. With starvation or insufficient intake of proteins from food, the albumin content in the plasma drops, which can lead to increased accumulation of water in the tissues (edema). This condition associated with protein deficiency is called starvation edema [26]. As a result of the conducted studies, there is an increase in this indicator after feeding in all experimental variants and in the control group, there are no significant differences among these groups (p > 0.05).
The data obtained during the study indicate that the use of astaxanthin in concentrations of 20.0, 30.0, 40.0 and 50.0 mg gives the best physiological and biochemical parameters of cultured individuals. However, taking into account the maximum input of astaxanthin into feed for sturgeon fish species, the most effective and safe concentration of astaxanthin in the diet of valuable aquaculture objects is 40.0 mg/kg of feed, which is confirmed by the results obtained during the experiments.
Thus, the results obtained indicate the effective utilization of consumed feed and activation of plastic metabolism, which is confirmed by the data of physiological and biochemical analysis, as well as the absence of any disturbance in the transformation of substances in the body. This indicates the effectiveness of the use of astaxanthin in sturgeon feed, the highest physiological and biochemical parameters were characteristic of Test 3, where astaxanthin was added to the feed 40 mg per 1 kg of feed. The indicators of energy metabolism also indicate a better accumulation of plastic substances in the fish of this experimental group, the level of total protein was significantly higher by 26.4% (р ≤ 0,05) compared to the control sample, the concentration of hemoglobin was 77.4 g/l, which is 17.6% higher than in the fish of the control group.
Carotenoids, being precursors of retinol, play an important role in the antioxidant system [27, 28]. These properties are determined by the structural features of these substances. The presence of a large number of double bonds in carotenoid molecules helps to reduce the aggressive effects of free radicals, and thereby protects biological membranes from damage. It should be noted that the inclusion of synthetic astaxanthin in the compound feed for sturgeon negatively affects the growth and survival of fish, compared with natural carotenoid preparations [29]. It is a well-known fact that astaxanthin has a much greater antioxidant property compared to other carotenoids. This is due to the presence of hydroxyl groups in its molecules. However, this statement concerns only astaxanthin of natural origin, in this study natural (natural) astaxanthin was used whereas preparations from its synthetic analogue have the same chemical formula, but in a different isomeric form. Probably, this is the reason for the insufficient effectiveness of this additive in the cultivation of sturgeon fish.
Due to the fact that the natural antioxidant astaxanthin has pro-A-vitamin activity [30], it was advisable to determine the probability of accumulation of vitamin A and carotenoid in the liver. Vitamin A or retinol is found exclusively in animal fats (in the study, liquid fish oil was used, which contains vitamin A, natural astaxanthin was dissolved in it).
The use of compound feed with astaxanthin had a significant effect on the content of carotenoids in the liver. Before the experiment, the carotenoid content in the liver of hydrobionts was no higher than 0.73 mg per 100 g of raw tissue, after feeding, the indicators changed markedly, in the experimental variants an increase in the carotenoid content in the liver was noted from 1.09-1.78 mg per 100 g of raw tissue, while in the control this indicator changed by 0.37 mg per 100 g of raw fabric. In the experimental versions, this value was 2.0 times higher (Fig. 5).

Fig. 5. Biochemical parameters of sterlet liver (amount of carotenoid) (n = 16)
The most carotenoid in the liver was observed in Tests 3 and 4, the indicators in these study groups were higher by 1.31-1.37 mg per 100 g of raw tissue, in contrast to the control sample.
A similar situation turned out to be with the amount of vitamin A in the liver, at the beginning of the experiment, the indicators of the study Tests were of the same order (p < 0.05) (Fig. 6).
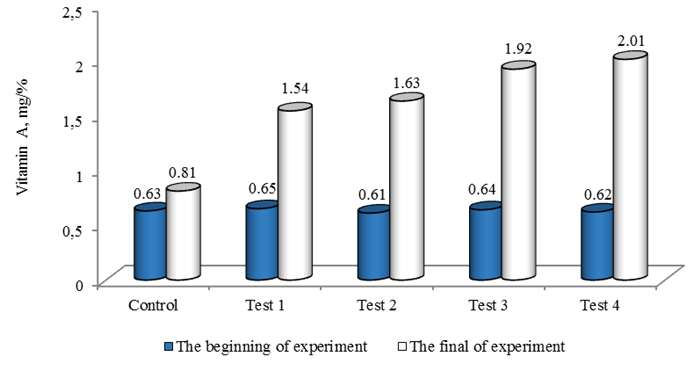
Fig. 6. Biochemical parameters of sterlet liver (amount of vitamin A) (n = 16)
Upon completion of feeding, the fish that consumed feed, with the addition of astaxanthin, the indicators for the amount of vitamin A accumulation changed significantly compared to the control group. The indicators of Tests 1, 2, 3 and 4 were 1.9, 2.0, 2.4 and 2.5 times higher, respectively, in comparison with the control sample.
According to literature data, the content of vitamin A in the liver of sturgeon fish varies widely and depends on the characteristics of nutrition, age, sex, place of catch, even for the same type of fish, the concentration of vitamin A in the liver varies depending on the conditions of its existence, and increases significantly with the age of the fish. For juvenile sturgeon species, this indicator varies from 0.5-29.4 mg/%, for older age groups from 2.5-131.3 mg/% [31].
As a result of the experiment, it was found that the accumulation of vitamin A in the tissues of farmed fish occurs in proportion to the increase in carotenoid, this conclusion is confirmed by the opinion of other authors [32, 33]. This suggests that the addition of natural astaxanthin in combination with liquid fish oil to the compound feed provides an increase in the concentration of vitamin A in the liver of fish, which is a favorable sign of good fish health.
Thus, the conducted studies have generally shown that the use of natural astaxanthin in the composition of feed for sturgeon fish is effective, leads to an improvement in the physiological state of cultivated hydrobionts. This indicates that astaxanthin is of natural origin, performs antioxidant and anti-stress functions.
Conclusion
The study of the most effective concentration of astaxanthin in the diet of valuable fish species revealed that the use of astaxanthin of natural origin in concentrations of 20.0, 30.0, 40.0 and 50 mg per 1 kg of feed gives the best physiological and biochemical indicators of cultured individuals in all experimental versions of the study. This is quite understandable, according to the literature, the antioxidant astaxanthin of natural origin has a unique molecular structure that allows it to be both inside and outside the cell membrane at the same time. The mechanisms of action of astaxanthin in protection against oxidative damage include suppression of singlet oxygen, absorption of radicals to prevent chain reactions, inhibition of peroxidation to preserve the membrane structure, improvement of immune system function and regulation of gene expression. As a result of the conducted studies, it was found that the most effective input rate for sturgeon fish species is a dosage of 40.0 mg/kg.
Indicators of energy metabolism and hematological indicators indicate a better accumulation of plastic substances in the fish of the experimental group (a variant with the addition of astaxanthin to feed 40 mg/kg).
Hemoglobin in this sample was 17.6% higher than in the fish of the control group, in the same version of the study, in comparison with the control group, the level of total protein was significantly higher by 26.4% (p < 0.05). At the end of the experiment, in the experimental variants, the glucose concentration was within the physiological norm (from 3.0-4.0 g/l), which is the result of the normal operation of the enzymatic system that catalyzes the transformation of glucose, the control group was characterized by low glucose concentrations below the reference values.
As a result of the conducted studies, it was found that the accumulation of carotenoid and vitamin A in the tissues of farmed fish occurs in proportion to the increase in carotenoid. This suggests that the addition
of natural astaxanthin to the compound feed provides an increase in the concentration of vitamin A in the liver of fish, which is a favorable sign of good fish health.
Thus, it can be said that the antioxidant astaxanthin provided more favorable trophic and biochemical conditions necessary, in particular, for the normal growth and development of fish. The fish during the experiment with astaxanthin did not show any anxiety after feeding. Astaxanthin did not show a pigmenting role in the coloring of tissues and external integuments on sterlet. The results obtained indicate the effective utilization of consumed feed and activation of plastic metabolism, which is confirmed by the data of physiological and biochemical analysis, as well as the absence of any disturbance in the transformation of substances in the body.
1. Хохлова Н. Ф. Тенденции развития рыбоводства и рыболовства в России // Вестн. Моск. финансово-юрид. ун-та. 2021. № 4. С. 109–119.
2. Пономарев С. В., Бахарева А. А., Грозеску Ю. Н. Корма и кормление рыб в аквакультуре. М.: Моркнига, 2013. 417 с.
3. Akhmedzhanova A. B., Ponomarev S. V., Fedorvykh Yu. V., Levina О. А., Terganova N. V. Antioxidant astaxanthin in composition of sturgeon feeds // Vestnik of Astrakhan State Technical University. Series: Fishing industry. 2023. N. 1. P. 55–63. (In Russ.). https://doi.org/10.24143/2073-5529-2023-1-55-63.
4. Пономарев С. В., Пономарева Е. Н. Каротиноиды в аквакультуре осетровых рыб. Ростов н/Д.: Изд-во ЮНЦ РАН, 2010. 148 с.
5. Грозеску Ю. Н. Инновационные методы повышения эффективности кормления осетровых рыб на основе использования в рационах нетрадиционного кормового сырья и биологически активных препаратов: автореф. дис. ... д-ра с.-х. наук. Усть-Кинельский, 2016. 34 с.
6. Грозеску Ю. Н., Митрофанова М. А. Новый каротиносодержащий препарат с составе комбикормов для осетровых рыб // Вестн. Астрахан. гос. техн. ун-та. Сер.: Рыбное хозяйство. 2004. № 2 (21). С. 81–88.
7. Карнаухов В. Н. Функции каротиноидов в клетках животных. М.: Наука, 1973. 104 с.
8. Карнаухов В. Н., Федоров Г. Г. Каротиноиды в адаптации клеток животных к высокогорной гипоксии. Пущино: Наука, 1982. 42 с.
9. Gzeczuga B. Comparative studies of the occurrences and xanthophylls in reproductive cells of water animals // Folia Histochem. et cytochem. 1973. V. 11. P. 275–286.
10. Поддубная И. В. Кормление рыб. Саратов: Изд-во Саратов. ГАУ, 2016. 91 с.
11. Колб В. Г., Камышников В. С. Клиническая биохимия: пособие для врачей-лаборантов. Минск: Беларусь, 1976. 311 с.
12. Филиппович Ю. Б., Егорова Т. А., Севастьянова Г. А. Практикум по общей биохимии. М.: Просвещение, 1975. 318 с.
13. Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor // Clinic Chemistry Acta. 1969. V. 6. P. 24–25.
14. Fish bach F., Dunning M. A manual of laboratory diagnostic tests. Lppincott Williams & Wilkins, 2004. 1291 p.
15. Ледвина М. Определение β-липопротеинов сыворотки крови турбидиметрическим методом // Лабораторное дело. 1960. № 3. С. 13.
16. Van Kampen E. J., Zjilstra W. G. Standardization of hemoglobinometry. The hemiglobincyanide method // Clin. Chim. Acta. 1961. Р. 538.
17. Лиманский В. В., Яржомбек A. A., Бекина E. H., Андронников С. Б. Инструкция по физиолого-биохимическим анализам рыб. М.: Изд-во ВНИИПРХ, 1984. 59 с.
18. Иванова Н. Т. Атлас клеток крови рыб. М.: Лег. и пищ. пром-сть, 1983. 184 с.
19. Карнаухов В. Н. Методы определения содержа-ния каротиноидов и витамина А в тканях животных. Пущино: Наука, 1982. 28 с.
20. Лакин Г. Ф. Биометрия. М.: Высш. шк., 1990. 293 с.
21. Гераскин П. П., Ковалева А. В., Григорьев В. А., Фирсова А. В., Яицкая М. В., Ветрова В. Ж. Оценка физиологической подготовленности к репродуктивной функции доместицированных самок и выращенных от икры в искусственных условиях // Вестн. Астрахан. гос. техн. ун-та. Сер.: Рыбное хозяйство. 2019. № 4. С. 95–103.
22. Матишов Г. Г., Кокоза А. А., Металлов Г. Ф., Гераскин П. П. Комплексный подход к проблеме сохранения и воспроизводства осетровых рыб Каспийского моря. Ростов н/Д.: Изд-во ЮНЦ РАН, 2017. 352 с.
23. Пронина Г. И., Корягина Н. Ю. Референсные значения физиолого-иммунологических показателей гидробионтов разных видов // Вестн. Астрахан. гос. техн. ун-та. Сер.: Рыбное хозяйство. 2015. № 4. С. 103–108.
24. Пономарева Е. Н., Сорокина М. Н., Григорьев В. А., Ковалева А. В., Корчунов А. А. Результаты разработки методов формирования маточных стад стерляди в условиях замкнутого водообеспечения // Вестн. Астрахан. гос. техн. ун-та. Сер.: Рыбное хозяйство. 2010. № 1. С. 86–90.
25. Сементина Е. В., Серпунин Г. Г. Рыбоводно-биологическая и гематологическая характеристика ремонтно-маточного стада стерляди, выращиваемой в установках замкнутого водоснабжения // Рыбное хозяйство. 2011. № 4. С. 76–78.
26. Гулиев Р. А., Мелякина Э. И. Некоторые биохимические показатели крови рыб дельты Волги // Вестн. Астрахан. гос. техн. ун-та. Сер.: Рыбное хозяйство. 2014. № 2. С. 85–91.
27. Miki W. Biologocal function and activities of animal carotenoids // Pure and Appl. Chem. 1991. V. 63. N. l. P. 141–146.
28. Palozza P., Krinsky N. Antioxidant effects of carotenoids in vivo and in vitro // Мethods in enzymology. 1992. V. 213. P. 403–420.
29. Ponomarev S., Bahareva A., Puzankov I., Harlamova J., Mitrifanova M. Use of carotene contaning medicinal preparations to increase starlet early fry viability // Poster of 5th International Symposium on sturgeon, Iran. Ramsar, 2005. P. 80–81.
30. Душейко А. А. Витамин А. Киев: Наук. думка, 1988. 104 с.
31. Колчев В. В. Печень некоторых промысловых рыб водоемов СССР как основной источник витамина А // Тр. Всесоюз. науч.-исслед. ин-та мор. рыб. хоз-ва и океанографии. 1952. Т. 20. С. 170–176.
32. Абросимов С. С. Рост и развитие молоди русского осетра в связи с обеспеченностью стартового корма каротиноидами: автореф. дис. ... канд. биол. наук. М., 1992. 24 с.
33. Остроумова И. Н. Биологические основы кормления рыб. СПб.: Изд-во ГосНИОРХ, 2001. 372 с.





















