Россия
Исследована морфологическая структура органов пищеварительной системы (желудок, кишечник и пищеварительная железа) австралийского красноклешневого рака (Cherax quadricarinatus) при использовании муки из люцерны в составе кормов в качестве альтернативной добавки. Раков содержали в лабораторных условиях, при температуре 24–26,5 °С и делили на 3 группы: контроль (11 особей); раки, получавшие экспериментальный вариант корма № 1 (10 особей) и вариант корма № 2 (10 особей). Контрольную группу кормили гранулированным кормом, предназначенным для осетровых рыб. В экспериментальных кормах рыбная мука на 10 % была заменена на муку из люцерны. В вариант корма № 1 был добавлен ферментный препарат натузим в количестве 350 мг/кг корма. Эксперимент длился 45 суток. Кормление проводили 2 раза в сутки из расчета 2–4 % от массы тела. На гистологических препаратах контрольных и экспериментальных особей повреждений слизистой оболочки желудка выявлено не было. Толщина стенки желудка от мышечного слоя до края эпителия колебалась в широком диапазоне – от 40 до 200 мкм, независимо от экспериментальной группы. В слизистой оболочке не было отмечено больших скоплений гемоцитов, что свидетельствует об отсутствии воспалительного процесса. Толщина эпителиального слоя кишечника составляла 40–55 мкм. Наблюдалось большое количество бокаловидных клеток, что может свидетельствовать об активно идущем процессе пищеварения. Патологических изменений в строении кишечника выявлено не было. Поперечный размер трубочек пищеварительной железы был в диапазоне от 180 до 300 мкм. На поперечном срезе доля железистой части трубочек достигала 80–90 %. Микропрепараты пищеварительной железы раков, получавших экспериментальные корма, морфологически не отличались от контрольной группы.
австралийский красноклешневый рак (Cherax quadricarinatus), мука из люцерны, натузим, желудок, пищеварительная железа, кишечник
Introduction
Australian redclaw crayfish (Cherax quadricarinatus) is a representative of the order of decapod crustaceans and as an object of aquaculture it is actively grown in farms. The diet plays an important role in the physiological state of crayfish [1]. At the same time, there are no recipes and industrial production of compound feeds for this type of crustaceans in Russia. When growing crustaceans, it is necessary to use feed intended for other aquatic organisms, such as sturgeon or salmon [2, 3]. However, this is not economically justified.
The Australian crayfish is an omnivorous species, which makes it possible to include a wide range of ingredients of animal and vegetable origin in the composition of feed during its commercial cultivation [4]. Previous studies on the possibility of replacing fishmeal with various vegetable sources of protein and lipids have shown that diets containing inexpensive plant ingredients are suitable for Australian redclaw crayfish, without compromising survival and growth [5].
Vegetable feed additives are a source of readily available nutrients [6], especially in the diet of crustaceans, since most of their diet consists of plant food. Alfalfa flour contains up to 25% of easily digestible nitrogenous substances with the entire spectrum of essential amino acids, as well as carotene, vitamins D, E and B, a large amount of potassium and calcium. Using plant components as feed additives can significantly reduce the cost and improve the quality of aqua-culture products. At the same time, the issue of assessing the effect of these additives on the organs of the digestive system remains urgent. Despite the fact that crustaceans are a fairly common object of aquaculture, the literature available to us contains very little information about the microscopic structure of their organs and tissues, especially under the influence of various factors.
The aim of this work was to study the morphological structure of the digestive system of Australian redclaw crayfish when using alfalfa flour in feed as an alternative additive.
Materials and methods
Experimental studies were carried out on the basis of the scientific laboratory of ichthyopathological studies and complex approbation of preparations of Astrakhan Astrakhan Tatishchev State University and the Laboratory of physiology of fish nutrition of Astrakhan State Technical University.
The object of the study was an Australian redclaw crayfish (Cherax quadricarinatus) with an average weight of 36.5 g, in an amount of 31 individuals. Crayfish were kept in aquariums with a volume of 150 liters at a temperature of 24-26.5 °C. Crayfish were divided into 3 groups: control (11 individuals); crayfish receiving experimental version of feed No. 1 (10 individuals); crayfish receiving experimental version of feed No. 2 (10 individuals).
The control group was fed with granular feed intended for sturgeon fish. The feed consisted of the following components: fish flour, blood, meat, meat and bone, wheat germ, gluten, grain and meal extrudates, minerals, vitamins, fish oil, bioflavonoids, antioxidants, vegetable oil, phytase.
Based on the literature data on nutritional needs, nutritional value and the use of various components in the composition of compound feeds for Australian redclaw crayfish [7], two formulations of experimental feeds were developed. Composition of the feed was selected so as to reduce the cost of the feed as much as possible. The formulations included components of animal and vegetable origin in different percentages: fish meal, fish oil, wheat flour, alfalfa flour, corn gluten, boiled carrots, calcium, premix.
In experimental feeds, fishmeal was replaced by alfalfa flour by 10%. An enzyme preparation – natusim in the amount of 350 mg per 1 kg of feed was added to the experimental version of feed No. 1.
Preparation of experimental batches of feed included the following stages: preparation of components, their grinding, production of granules by wet pressing, drying in a dryer, application of fat by vacuum spraying. Finished pellets of 4-4.5 mm in size, after vacuum application of fat on them, were a completely finished product, which was fed to crayfish. The analysis of the compound feed composition was carried out according to the scheme developed by other authors during zootechnical and biochemical analyses of feed for crustaceans [2].
The experiment lasted 45 days. Feeding was given 2 times a day. The feeding norm made 2-4% of body weight.
The effectiveness of the diets was evaluated by fish-biological (growth rate, survival rate) and histological indicators [8].
For histological analysis, the stomach was taken as an organ of the very first organ of the digestive system involved in digestion, the digestive gland taking an active part in the process of digestion and absorption, as well as the initial part of the intestine.
The tissues were fixed in a Buena mixture, filled with paraffin and sections with a thickness of 7 microns were made [9]. The obtained sections were stained with hematoxylin-eosin and photographed with an Olympus BX 53 microscope.
Results and discussion
Australian crayfish are omnivorous and unpretentious in food. Their diet includes vegetable foods and animal proteins. Crayfish can feed on aquatic plants, small representatives of the ichthyofauna. The digestive system begins with the mouth opening, then food enters the pharynx, short esophagus and stomach. The stomach is divided into two sections – chewing and filtering. Next, the food enters the middle intestine, where the ducts of the digestive gland open. Undigested residues enter the posterior intestine and are discharged through the anal opening on the tail blade.
Our previous studies have shown the possibility of partial replacement of fish meal with vegetable protein in the preparation of feed for growing in artificial conditions of Australian redclaw crayfish. The decrease in the amount of animal protein in the feed did not significantly affect the main fish-biological (survival rate, increase in body length and weight) and biochemical (content of protein, glucose, lipids, urea, etc. in the hemolymph) indicators of crayfish. There was a decrease in mortality and a gain in body length, especially when the enzyme natusim was added to the feed [10].
This article presents the results of histological studies of the digestive tract of the stomach, intestines and digestive gland with partial replacement of fish meal in the feed with vegetable protein.
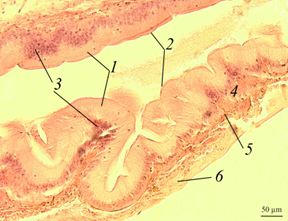
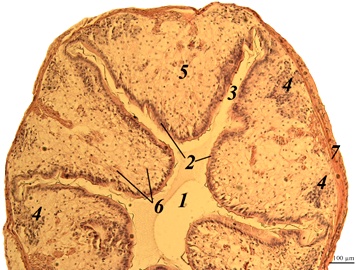
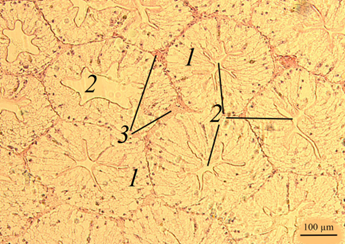
The stomach. The histological picture of the stomach of the redclaw crayfish of the control and experimental groups is shown in the figures (Fig. 1).
|
|
|
|
|
а
|
b
|
|
|
|
|
|
|
с |
d |
|
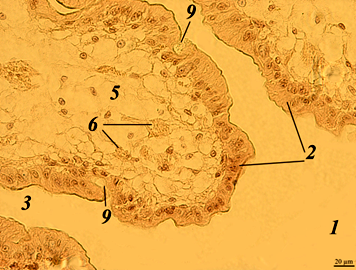
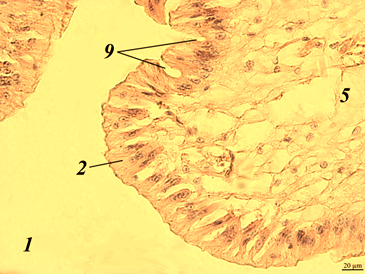
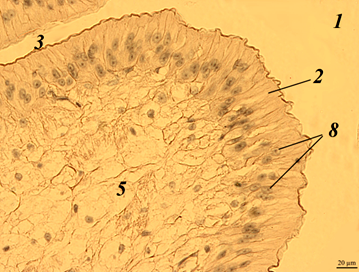
Fig. 1. The stomach of redclaw crayfish:
a – review preparation (control); b – stomach wall (control); c – stomach wall (feed option No. 1);
d – stomach wall (feed option No. 2): 1 – mucous membrane; 2 – mucous layer; 3 – glandular cells;
4 – submucosal layer; 5 – muscle layer; 6 – serous membrane
The stomach wall of crayfish is formed by a mucous membrane 1 covered with mucus 2, gastric glands 3, submucosal layer 4, muscle 5 and serous membrane 6. The wall thickness from the muscle layer to the edge of the epithelium ranged in a wide range – from 40 to 200 μm, even in the same individual, regardless of the experimental group.
The gastric mucosa had a folded structure, comb-shaped folds, rare, deepened folds sometimes branched. The inner surface of the mucous membrane is smooth. The surface of the folds is covered with a thin layer of mucus. At the base of the mucous membrane are the glands of the mucous membrane. The mucous membrane is bounded from the submucosal base by a thin muscular layer.
No damage to the gastric mucosa was detected on the studied histological preparations of control and experimental individuals of redclaw crayfish. Also, there were no large accumulations of hemocytes in the area of the mucous membrane, which could indicate the development of an inflammatory process.
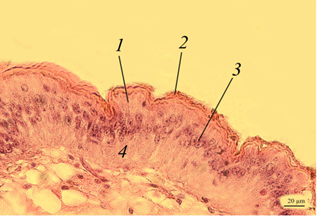
The intestine. The morphological picture of the intestine of red-lobed crayfish of the control (Fig. 2, a, b) and experimental groups (Fig. 2, c, d) did not differ significantly.
|
|
|
|
a
|
b
|
|
|
|
|
c
|
d |
Fig. 2. The intestine of redclaw crayfish:
a – review preparation (control); b – intestinal epithelium (control); c – intestinal epithelium (feed variant No. 1);
d – intestinal epithelium (feed variant No. 2); 1 – intestinal lumen; 2 – edged epithelium; 3 – crypt; 4 – immune cells;
5 – connective tissue; 6 – blood vessels; 7 – muscle layer; 8 – nucleus of epithelial cells; 9 – goblet cell
The inner surface of the intestine had a folded structure with deep crypts 3 and was lined with edged epithelium 2. At the base of the folds are clusters (follicles) of immunocompetent hemocytes 4. Such clusters are very similar to the lymphoid tissue associated with the intestine, which is observed in fish and amphibians. It is possible that in crayfish they are formed by vascular extensions and filled with hemocytes.
Enterocytes of the epithelium are prismatic in shape, tightly adjacent to each other. In enterocyte cells, the nuclei 8 had a rounded or oval shape, mainly with a single nucleolus. The thickness of the epithelial layer was 40-55 μm.
Thus, the microscopic structure of the organ indicates the full functioning of its main cellular structures both in the case of using traditional feed, and when using both variants of experimental feeds.
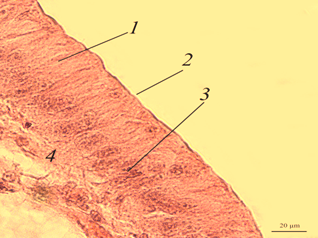
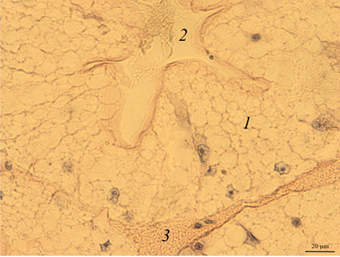
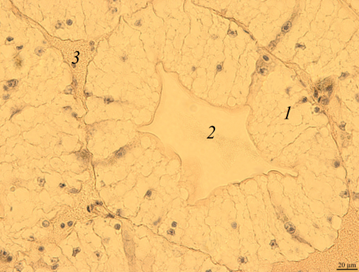
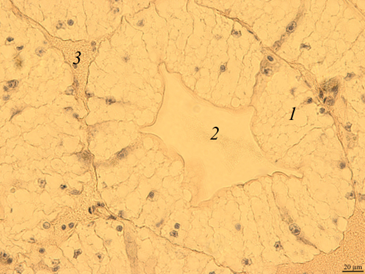
Digestive gland. The digestive gland of redclaw crayfish is represented by a system of branched tubules. The tubes are packed parallel to each other and have a rounded or oval shape on the cross-section (Fig. 3, a, b).
|
|
|
|
а
|
b
|
 |
|
|
с
|
d
|
Fig. 3. The digestive gland of redclaw crayfish:
a – review preparation (control); b – the cross-section of the digestive gland tube (control); c – the cross-section
of the digestive gland tube (feed option No. 1); d – the cross-section of the digestive gland tube (feed option No. 2);
1 – glandular tissue (cells of the digestive gland); 2 – the duct of the digestive gland; 3 – lacunae filled with hemolymph
The transverse size of the tubes usually ranges from 180 to 300 μm. Glandular tissue 1 is formed by strands of cells, in the cytoplasm of which optically bright areas are detected. The reason for this phenomenon is not completely clear. These may be digestive enzymes, and it cannot be excluded that light areas may occur as a result of the leaching of glycogen from cells during histological treatment of drugs. The inner part of the tube of the digestive gland has a folded structure. Folding in different tubes can be expressed to varying degrees. In the middle part of the tubules there is a duct 2 that removes digestive enzymes into the intestinal cavity. There are gaps filled with hemolymph at the contact points of the tubes 3.
On the histological preparation, the nuclei of glandular cells are clearly visible, usually having a rounded shape with one well-distinguishable nucleolus in the middle. On the cross-section, the proportion of the glandular part of the tubules reached 80-90%.
Micro-preparations of the digestive gland of redclaw crayfish receiving experimental feeds (Fig. 3, c, d) practically did not differ from the control group.
In the shape, size of the tubes of the digestive gland, the structure of their glandular part, the histological picture was identical in all the studied groups. Infiltration by immune cells, which is an indicator of inflammatory and necrotic processes, was not detected.
Thus, the partial replacement of fish meal with a vegetable component did not lead to pathological changes in the structure of the studied organs of the digestive system. Most likely, this is due to the omnivorous nature of the redclaw crayfish and the adaptability of its digestive system to eating and digesting both plant and animal food.
Conclusion
The results of the conducted studies made it possible to assess the safety of partial replacement of fish meal with vegetable protein for the digestive system of Australian redclaw crayfish when preparing feed for its cultivation in artificial conditions.
No mucosal damage was detected on the studied histological preparations of the stomach, and there were also no signs of the development of the inflammatory process.
The morphological state of the intestine, namely the integrity of the apical surface of enterocytes and the presence of a large number of goblet cells indicates a good course of the digestive process.
There were no signs of pathological processes (in the form of necrosis and inflammation) in the glandular part of the digestive gland. The tubes of the digestive gland did not differ significantly in their dimensional characteristics in individuals of different experimental groups.
Thus, the complex of biochemical, physiological, fish-biological and morphological studies performed by us indicates that the developed feed formulations are able to positively change the functional parameters of the organism of the redclaw crayfish (Cherax quadricarinatus), but during the studied period there are no pronounced structural changes in the organs and tissues of the digestive system.
1. Rodríguez-González H., García-Ulloa M., Her-nández-Llamas A., Villarreal H. Effect of dietary protein level on spawning and egg quality of redclaw crayfish Cherax quadricarinatus // Aquaculture. 2006. V. 257. N. 1. Р. 412-419.
2. Лагуткина Л. Ю., Мартьянов А. С., Степанов Р. В., Шейхгасанов К. Г. Оптимизация технологии кормления австралийских раков с помощью рецептур экспериментальных кормов // Вестн. Астрахан. гос. техн. ун-та. Сер.: Рыбное хозяйство. 2016. № 1. С. 77-87.
3. Борисов Р. Р., Ковачева Н. П., Артемов Р. В., Никонова И. Н., Арнаутов М. В., Артемов А. В., Гершунская В. В. Оценка эффекта применения комбикормов с различным уровнем белка для молоди австралийского красноклешневого рака в условиях УЗВ // Тр. ВНИРО. 2022. Т. 187. С. 128-137.
4. Jones C. M. The biology and aquaculture potential of the tropical freshwater crayfish Cherax quadricarinatus. Queensland Department of Primary Industries Information Series, 1990. 109 р.
5. Saoud I., Yta A., Ghanawi J. A review of nutritional biology and dietary requirements of redclaw crayfish Cherax quadricarinatus (von Martens 1868) // Aquaculture Nutrition. 2012. V. 18. P. 349-368.
6. Костомахин Н. А., Иванов А. И. Травяная мука -белковый и витаминный корм // Корма. 2013. № 6. С. 71-73.
7. Гобелков П. В. Темп роста самцов австралийского красноклешневого рака (Сherax quadricarinatus) в условиях индустриальной аквакультуры при использовании кормов различного состава // Биологическое разнообразие: изучение, сохранение, восстановление, рациональное использование: материалы II Междунар. науч.-практ. конф. (Керчь (27-30 мая 2020 г.)). Симферополь: ООО «Издательство Типография «Ариал», 2020. С. 283-287.
8. Пономарев С. В., Гамыгин Е. А., Никоноров С. И., Пономарева Е. Н., Грозеску Ю. Н., Бахарева А. А. Технологии выращивания и кормления объектов аквакультуры юга России. Астрахань; М.: Нова плюс, 2002. 264 с.
9. Микодина Е. В., Седова М. А., Чмилевский Д. А., Микулин А. Е., Пьянова С. В., Полуэктова О. Г. Гистология для ихтиологов: опыт и советы. М.: Изд-во ВНИРО, 2009. 112 с.
10. Ширина Ю. М., Файзулина Д. Р., Конькова А. В., Котельников А. В., Богатов И. А., Котельникова С. В. Перспективы использования муки из люцерны в составе кормов для австралийского красноклешневого рака (Cherax quadricarinatus) // Вестн. Астрахан. гос. техн. ун-та. Сер.: Рыбное хозяйство. 2023. № 1. С. 72-81.