Россия
Россия
Представлено исследование по подбору оптимального состава протекторной среды для криоконсервации репродуктивных клеток самцов африканского клариевого сома. В качестве основных криопротекторных добавок изучены глицерин, диметилсульфоксид (ДМСО) в концентрациях 3, 5 и 10 %. Проведена оценка влияния данных соединений на показатели качества спермы сома (процент и время движения спермиев после активации), уровень пероксидации липидов спермы и активность антиоксидатного фермента каталазы до и после процесса криоконсервации. Установлено снижение рыбоводных показателей качества спермы африканского сома (доля живых клеток, время подвижности) при добавлении ДМСО в базовую криосреду. Наибольшее снижение процента подвижности спермиев до замораживания наблюдается при внесении ДМСО в концентрации 10 %. Отмечен дозозависимый эффект снижения активности спермы после замораживания при увеличении добавки ДМСО в криосреду с 3 до 10 %. Показано, что 3 % глицерина в криосреде не способствует сохранению клеток при замораживании; криоповреждения, вызванные образованием кристаллов льда, приводят к значительному снижению подвижности спермы сома. Лучшие показатели качества спермы получены при использовании глицерина, наиболее эффективная концентрация составляет 5 % (40 % подвижных репродуктивных клеток в течение 53 с). Добавка 5 % глицерина в криосреду способствует наибольшему снижению уровня пероксидации липидов спермы и повышению активности каталазы, что полностью согласуется с результатами по подвижности сперматозоидов. Рассчитанные корреляционные коэффициенты подтверждают зависимость репродуктивных качеств гамет сома от антиоксидантного статуса клеток. Полученные результаты свидетельствуют о важности использования антиоксидантов в качестве криопротекторных добавок, улучшающих рыбоводные показатели качества спермы африканского сома, и указывают на необходимость проведения дополнительных исследований по оценке влияния новых потенциально эффективных и безопасных антиоксидантов в качестве криозащитных добавок.
аквакультура, криоконсервация, репродуктивные клетки, криопротектор, африканский клариевый сом, искусственное воспроизводство, биотехнология
Introduction
Aquaculture as a rapidly growing agricultural sector is of great importance for food security and economic stability worldwide [1]. Catfish (Siluriformes) make up the largest order of a bony fish with a growing production volume in many countries of Africa, South America and Europe [2]. The African sharptooth catfish (Clarias gariepinus, Burchell, 1822) bred both inside and outside its natural rangeis known for its resistance to disease and stress, high growth rate, good adaptation to a wide range of environmental parameters, and tolerance for high stocking densities during artificial cultivation [3]. In addition, the African catfish is widely used as a model organism in a number of toxicological, morphological, and physiological studies [4, 5]. In the southern regions of Russia sharptooth catfish are grown in open reservoirs of pond farms during the warm period of the year, where it reaches a commercial mass in 5-6 months [3], they are also grown all year round in recirculating water systems [6].
In the natural habitat catfish have an intermittent annual reproductive cycle and their breeding season correlates with the periods of maximum rainfall [7], the best quality sperm is taken during the period of natural spawning [8]. When keeping catfish in artificial conditions, the period of their spawning does not always coincide with the technological process of the enterprise, as a result, the quality of the resulting sperm is significantly reduced. Growing catfish for a long time in closed systems is an additional stress factor for fish leading to a decrease of hybrid catfish production, high morbidity, and growth retardation [9]. In connection with these factors, cryopreservation of the reproductive cells of male fish is one of the main ways to solve the problems associated with the conservation of species diversity, improving the quality of the original genetic material, and providing the fish farm with high-quality sperm [10].
It is known that the success of cryopreservation largely depends on the freezing protocol used and the selection of the optimal cryoprotectant [11], since both of these factors significantly determine the degree of spermatozoa cryopreservation during intracellular ice crystallization [12]. African catfish sperm was first successfully cryopreserved by Steyn et al. [13] who obtained 40% sperm motility after 24 hours of cell storage in liquid nitrogen. Later it was shown that a medium containing glucose with dimethyl sulfoxide (DMSO) was effective [14]. In works [15] on the cryopreservation of African catfish sperm, cryoprotective medium components such as DMSO, ethanol, methanol, and glycerol were used, but glucose and glycerol remain the most widely used cryoprotectants. More recent studies have tested DMSO, glycerol, and methanol at concentrations ranging from 5 to 25% as cryoprotectants using various diluents and have shown that the combination of DMSO (10%) in Fish-Ringer Extender (NaCl (0.75 g), KCl (0.10 g), CaCl2 (0.016 g), MgSO4 (0.023 g), NaH2PO4 (0.041 g) and Glucose (0.10 g) per 100 ml) showed the highest mobility values. spermatozoa after thawing [12].
Despite the successes achieved in the cryopreservation of African catfish reproductive cells the results obtained are often not reproducible and do not always provide a high survival rate of defrosted spermatozoa. This is due to the fact that the cryopreservation process is influenced by a large number of factors: the physiological state of spawners, the quality of reproductive cells, the individual characteristics of fish from different populations, physical factors, etc. [16]. In this regard, at present, an important area of research is the adaptation of cryopreservation technologies for clariid catfish sperm developed in countries with its natural habitat, for the conditions of fish farms in southern Russia, by selecting the optimal composition of the cryoprotective environment [17]. The purpose of this study is to evaluate the effect of glycerol and DMSO supplementation at various concentrations as components of a cryoprotective medium for the successful preservation of the reproductive cells of male African catfish (Clarias gariepinus, Burchell 1822).
Material and methods
Collection of biomaterial. The material of research was the African catfish sperm (Clarias gariepinus, Burchell, 1822) selected from three males weighing from 3 to 3.5 kg. Spawners were harvested at a fish-breeding enterprise in Rostov-on-Don where they were kept in a recirculating water supply at a temperature
of 25 °C. To obtain reproductive cells a hormonal injection was previously carried out by the carp pituitary gland at a dose of 3 mg per 1 kg of body weight. Due to the biological characteristics of the reproductive system of males the testes were obtained by slaughter with a preliminary stunning of the fish with a blow
to the head. Fifteen hours after the pituitary injection, the body cavity was carefully opened with a sterile scalpel so as not to damage the testes and removed. Next, the testes were placed in a Petri dish and several incisions were made to release the sperm. Sperm from three males was mixed in one container, collected in
a sterile syringe and the volume was measured.
Freezing and thawing. Glycerol and DMSO at concentrations of 3, 5 and 10% were studied as the main components of the cryoprotectant. The diluent also included NaCl (6.5 g/l), KCl (0.25 g/l), CaCl2 (0.2 g/l), NaHCO3 (2 g/l). The sperm with cryoprotectant was diluted in a ratio of 1 : 1 and left for equilibration for 15 min at a temperature of +4 °C. The resulting solution was dispensed into labeled cryovials with screw caps of 2 ml. Then the material was frozen using a two-stage freezing protocol: in a Planer programmable freezer to a temperature of –70 °C, followed by immersion of the samples in liquid nitrogen (–196 °C) for long-term storage. Samples were thawed in a water bath at 38 °C. The quality of sperm in each variant of the experiment was assessed before and after deep freezing; the percentage of spermatozoa with translational and oscillatory movements and the time of their movement were determined. The percentage of motile spermatozoa was determined using a Mikmed-5 binocular microscope with an HB-200 video eyepiece (LOMO, Russia) after adding river water to the sperm as an activating solution in a ratio of 1 : 1 000.
We used catfish sperm with an activity of 4 and 5 points determined by the Persov scale [18], the concentration of spermatozoa in the sperm (Goryaev's chamber) was 2.61 ∙ 1 010 cells/ml. Duration (total time of movement of spermatozoa, s) was determined as the time from activation to the cessation of movement of the last spermatozoa in the field of view of the microscope using a stopwatch. All measurements were carried out by one operator in triplicate.
Determination of the level of accumulation of TBA-dependent products in the semen of the African catfish. The intensity of lipid peroxidation (LPO) was judged by the accumulation of secondary carbonyl products giving a colored complex with thiobarbituric acid (TBARS) [19]. To 3.9 ml of a potassium chloride solution cooled to 4 °C, 100 µl of African catfish sperm, mixed in a 1 : 1 ratio with cryomedium was added before cryopreservation or after defrosting. Samples of 1 ml of the mixture were taken from the resulting homogenate into test tubes for further centrifugation. In the same test tubes, 50 μl of solutions of ascorbic acid and Mohr's salt, 0.5 ml of trichloroacetic acid solution were added. The tubes were placed for 10 min in a water bath at 37 °C, centrifuged for 10 min at 3 000 rpm. Then, 1 ml of the supernatant liquid was taken into clean test tubes, 0.5 ml of a thiobarbituric acid solution was added, the samples were placed in a boiling water bath for 10 min, and then they were cooled in ice water to room temperature. After cooling, 0.5 ml of chloroform was added to the samples to obtain a clear solution and centrifuged for 15 minutes at a speed of 3 000 rpm. The supernatant liquid was taken and the extinction
of the sample was measured on an SF-103 spectrophotometer at 532 nm relative to the control sample. All measurements were carried out in triplicate.
The calculation is carried out according to the formula: X = (E ∙ 1.5 ∙ 3.2) / (0.156 ∙ 1.0); where X is the content of TBARS in the initial homogenate, nmol/ml; E – sample extinction; 3.2 is the total volume of the studied samples, ml; 1.0 is the volume of the supernatant taken for the determination of TBARS, ml; 1.5 – sample volume, ml; 0.156 – extinction of 1 nmol TBARS in 1 ml at 532 nm.
Determination of catalase activity. The activity of the antioxidant enzyme catalase was determined from the rate of H2O2 decomposition. For the study 200 µl of a mixture of sperm and cryomedium (1 : 1) was taken before or after freezing, 800 µl of phosphate buffer (pH 7.4) was added, and the resulting mixture was centrifuged at 3 000 rpm for 10 min. The reaction mixture contained 100 µl of a 30 mM H2O2 solution and 100 µl of the supernatant. The reaction was carried out for 10 min, the concentration of H2O2 was measured using an SF-103 spectrophotometer (λ = 240 nm). For each variant, 3 parallel independent measurements were carried out. On the basis of the obtained spectrophotometric data the kinetic curves of H2O2 consumption were constructed, which were linearized in the coordinates of the first-order equation. The rate constants of the first-order H2O2 decomposition reaction were calculated from the slope of the constructed straight lines.
Statistical analysis. Data analysis was performed using Statistica software for Windows, version 9.0 (StatSoft, Inc.). Data were presented as mean ± standard deviation. Using Student's t-test the obtained parameters were analyzed; statistical significance was set at p < 0.05.
Results and its discussion
It is known that the main limitation of the use of potential cryoprotectants in deep freezing of living cells is their toxicity. Muchlisin et al. [8] studied three natural non-toxic cryoprotectants in their works: egg yolk, glucose and honey in three concentrations (5, 10 and 15%), DMSO was used as control. The results of fertilization and hatching of larvae showed that when using DMSO as the main protective component the highest quality thawed sperm was obtained in comparison with other variants of the experiment but a higher level of fertilization and hatching was noted with the addition of 10% egg yolk. It was shown in [20, 21] that a decrease in the amount of DMSO in the cryoprotective solution from 10 to 4% for Russian sturgeon and to 3% for beluga leads to an increase in the lifetime of defrosted sturgeon cells.
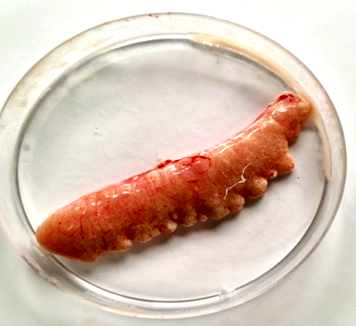
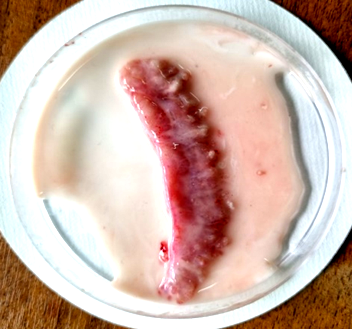
In this work we assessed the effect of glycerol and DMSO additives at concentrations of 3, 5 and 10% on the quality of reproductive cells of African catfish males before and after cryopreservation. The native catfish sperm obtained by incising the testicles (Fig. 1) has an activity of 100%, and the average life time of spermatozoa is 64 s.
a
b
Fig. 1. African catfish testis before (a) and after (b) incision
After equilibration of sperm together with a cryoprotector for 15 minutes, the greatest decrease in sperm activity is observed with the addition of 10% DMSO, where the proportion of living cells decreases to 61%. In other variants of the experiment, the percentage of motile spermatozoa is 95% or more (Table 1).
Table 1
Quality indicators of catfish sperm before and after the cryopreservation process
|
Сryoprotector |
Before freezing |
After thawing |
||
|
% mobility |
Lifetime, s |
% mobility |
Lifetime, s |
|
|
Native |
100,0 ± 1 |
64 ± 7 |
0 |
0 |
|
DMSO 3% |
96 ± 1 |
49 ± 3 |
6 ± 5 |
18 ± 14 |
|
DMSO 5% |
95 ± 5 |
47 ± 3 |
5 ± 3 |
25 ± 5 |
|
DMSO 10% |
61 ± 11 |
42 ± 2 |
1 ± 0 |
10 ± 1 |
|
Glycerin 3% |
98 ± 3 |
61 ± 5* |
0 |
0 |
|
Glycerin 5% |
95 ± 5 |
50 ± 2 |
40 ± 10 |
53 ± 4 |
|
Glycerin 10% |
99 ± 2* |
67 ± 6* |
21 ± 4 |
56 ± 5 |
* Average values for a series of experiments are given; differences from the control group p ≥ 0.05, for the rest differences from the control group p < 0.05; values are expressed as mean ± standard deviation.
The lowest life time in samples before freezing is also observed with the addition of 10% DMSO, the sperm motility time is 42 s, which is 1.5 times less than in native sperm. In variants with 5 and 3% DMSO sperm motility is higher and amounts to 47 and 49 s, respectively. In all experiments with glycerol, the lifetime of spermatozoa is higher than in experiments with DMSO, but less or comparable to the control without the addition of a cryoprotectant. The shortest lifetime in variants with glycerin is noted when it is added at a concentration of 5% (95%, 50 s). The addition of 10% glycerol to the composition of the cryoprotectant contributes to a slight increase in the lifetime of spermatozoa, by 3 s relative to the control (p > 0.05) (see Table 1).
After thawing in the control variant and in the sample containing 3% glycerol no active spermatozoa were found in the field of view. A low percentage of African catfish sperm activity (from 1 to 6%) is observed when DMSO is used at all concentrations; the life time of spermatozoa is from 10 to 25 s. The best result of cell motility (40%, 53 s) was obtained in the experiment with the addition of 5% glycerol. The use of glycerol at a concentration of 10% makes it possible to obtain 21% of living cells with a motility of 56 s after thawing (see Table 1).
It is known that deep freezing of fish reproductive cells leads to the development of oxidative stress which is accompanied by an imbalance between the formation and utilization of reactive oxygen species (ROS ) [22]. Due to the high content of polyunsaturated fatty acids in the lipid membrane of cell membranes, fish sperm is most susceptible to the toxic effects of ROS. The formation of a high level of ROS leads to an increase in the processes of LPO, proteins, inactivation of enzymes associated with sperm motility and DNA fragmentation [23]. Previously it was shown that the overproduction of ROS during the cryopreservation of fish sperm is one of the main causes of damage to vital biomacromolecules which in turn leads to a decrease in the quality
of germ cells [24].
In this regard the quality of African catfish sperm before the process of deep freezing and after thawing was assessed in terms of the level of accumulation of TBARS and the activity of the endogenous antioxidant enzyme catalase. After the equilibration stage an increase in the level of TBARS was found when 10% DMSO was added to the medium for which the lowest indicators of sperm quality before freezing were noted. A higher content of TBARS relative to the control is observed in the sample with 3% glycerol, despite its favorable effect on sperm survival and motility. The lowest intensity of the LPO process was found in samples containing 5% glycerol and 3% DMSO (1.37 and 1.39 nmol/ml, respectively) (Fig. 2: average values for a series of experiments are given).
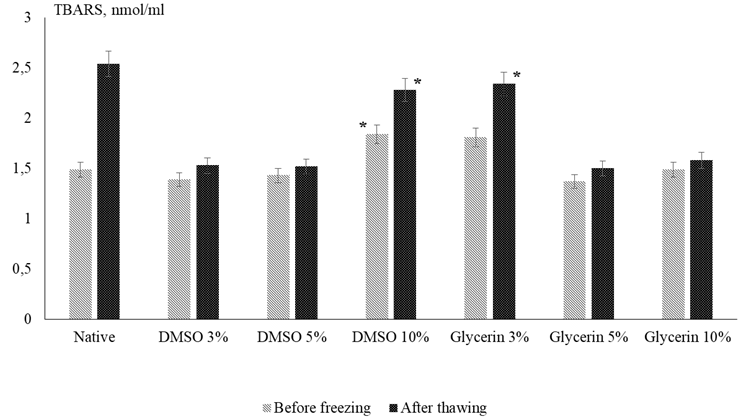
Fig. 2. Concentration of TBARS (nmol/ml) in samples before and after the cryopreservation process:
* differences from the control group p ≥ 0.05, for the rest differences from the control group p < 0.05;
values are expressed as mean ± standard deviation
The concentrations of TBARS in the variants with 5% DMSO and 10% glycerol did not differ statistically from the control (p > 0.05). After the thawing process, the content of TBARS naturally increases in all samples. After storage at the temperature of liquid nitrogen, all variants of the cryomedium modified by the addition of glycerol and DMSO contribute to a decrease in the LPO level of the catfish sperm in comparison with the control (see Fig. 2). The lowest efficiency of LPO reduction was noted when 10% DMSO (10% inhibition) and 3% glycerol (8% inhibition) were added to the medium; for the remaining variants, 38-41% inhibition of the accumulation of the TBARS level was observed. The addition of 3% glycerol and 10% DMSO had a lesser effect on lipid peroxidation without any noticeable antioxidant effect, which is in good agreement with the results on sperm motility.
Endogenous antioxidant enzymes of fish spermatozoa are the first line of protection of spermatozoa from the damaging effect of reactive oxygen species formed during gamete cryopreservation and have a preventive effect on radical oxidative processes, catalyzing the disproportionation reaction of the superoxide anion radical to hydrogen peroxide and molecular oxygen. The work showed that catalase sperm catalase activity regularly decreased in all experiments after thawing (Fig. 3: average values for a series of experiments are given).
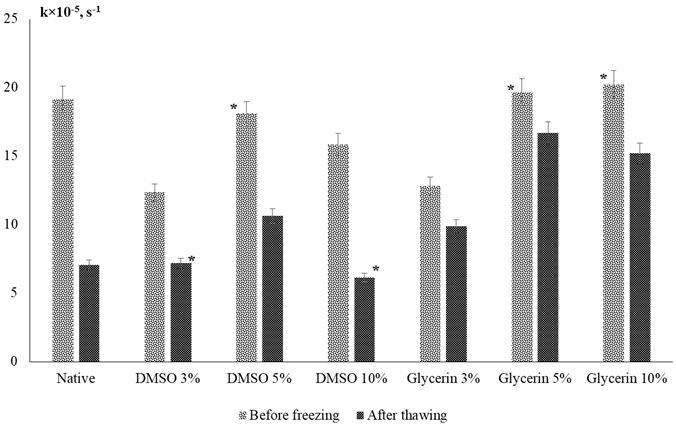
Fig. 3. Catalase activity in the presence of various concentrations of potential cryoprotectants before and after freezing:
* differences from the control group p ≥ 0.05, for the rest differences from the control group p < 0.05;
values are expressed as mean ± standard deviation
The highest rate of H2O2 decomposition prior to freezing was noted in the presence of 5 and 10% glycerol solutions, the lowest, with the addition of 3% glycerol and 3% DMSO. After defrosting, high protective activity is retained only for mixtures with the addition of 5 and 10% glycerol, for which the highest sperm motility after defrosting and a decrease in the concentration of TBARS in comparison with the control were established.
To establish the relationship between the quality indicators of the African catfish sperm before and after cryopreservation in the presence of the studied cryoprotectors, Pearson's correlation analysis was performed [25], the calculated coefficients are presented in Table 2.
Table 2
Pearson's correlation coefficients between African catfish sperm quality
and antioxidant activity before and after cryopreservation
|
Indicators |
Before freezing |
After thawing |
||||||
|
% live cells |
Lifetime, s |
TBARS, nmol/ml |
Catalase |
% live cells |
Lifetime, s |
TBARS, nmol/ml |
||
|
Before freezing |
Lifetime, s |
0.68 |
– |
– |
– |
– |
– |
– |
|
TBARS, nmol/ml |
–0.61 |
–0.07 |
– |
– |
– |
– |
– |
|
|
Catalase |
0.17 |
0.28 |
–0.42 |
– |
– |
– |
– |
|
|
After thawing |
% live cells |
0.23 |
0.04 |
–0.53 |
0.55 |
– |
– |
– |
|
Lifetime, s |
0.22 |
0.07 |
–0.56 |
0.60 |
0.88 |
– |
– |
|
|
TBARS, nmol/ml |
–0.26 |
0.26 |
0.70 |
–0.19 |
–0.63 |
–0.80 |
– |
|
|
Catalase |
0.42 |
0.28 |
–0.43 |
0.59 |
0.88 |
0.88 |
–0.60 |
|
Moderate positive correlations were established between the number of living cells and their lifetime before (r = 0.68) and after defrosting (r = 0.88). An interesting significant positive correlation (r = 0.88) was found between the activity of the antioxidant enzyme catalase and the number of living cells (r = 0.88), as well as their lifetime (r = 0.88) after thawing. Moderate negative correlations were established between the level of peroxidation of sperm lipids and the number of living cells before (r = –0.61) and after defrosting (r = –0.63), after thawing between the level of lipid peroxidation and the lifetime of sperm, catalase activity (r = –0.80 and r = –0.60, respectively).
It is worth noting that the revealed moderate patterns between the LPO level before and after freezing (r = 0.70) and catalase activity before and after thawing (r = 0.59). Interesting moderate dependences were also established between the activity of catalase before freezing and the lifetime of cells after thawing (r = 0.60). The obtained correlation coefficients confirm the dependence of sperm quality, primarily the number of living cells and their lifetime, on the antioxidant status of cells (the level of peroxidation of sperm lipids and the activity of the antioxidant enzyme).
Conclusion
Thus the negative effect of DMSO on the sperm of the African catfish, which manifests itself in the deterioration of fish breeding qualities (the proportion of living cells and the time of their motility) has been established. The addition of 10% DMSO to the cryomedium reduces the percentage of cell motility before freezing by 1.7 times. African catfish spermatozoa do not tolerate double temperature shock well, their activity naturally decreases from 6 to 1% as the concentration of DMSO in the cryogenic medium increases from 3 to 10%, respectively. When using glycerol, sperm survival rates are significantly better than when using DMSO. However 3% glycerol in the cryogenic medium is not enough to preserve cells during freezing. A low concentration of a potential protector is unable to protect cells from cryodamage as a result of the ice crystals formation with a decrease in temperature. For the reproductive cells of African catfish males it is optimal to use 5% glycerol as part of a cryoprotectant which makes it possible to obtain 40% of mobile reproductive cells within 53 seconds. The highest inhibitory and H2O2-utilizing activity was found for samples with the addition of 5% glycerol, which is in good agreement with the data obtained on sperm motility. The revealed patterns of the reproductive qualities of gametes of the soma on the antioxidant status of cells confirm the importance of using antioxidants as cryoprotectors and indicate the need for additional research on the effect of adding new potential inhibitors of radical processes as cryoadditives to basic media.
1. Gjedrem T., Robinson N., Rye M. The importance of selective breeding in aquaculture to meet future demands for animal protein: A review // Aquaculture. 2012. V. 350-353. P. 117-129. https://doi.org/10.1016/j.aquaculture.2012.04.008.
2. FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in action. Rome: Food and Agriculture Organization of the United Nations, 2020. 224 р.
3. Сидорова В., Асылбекова С., Январёва Н., Койшыбаева С., Бадрызлова Н., Мухрамова А., Шуткараев А. Экструдированные стартовые комбикорма для клариевого сома // Вестн. Астрахан. гос. техн. ун-та. Сер.: Рыбное хозяйство. 2020. № 2. С. 82-92. DOI: https://doi.org/10.24143/2073-5529-2020-2-82-93.
4. Nyang'ate Onura C., Van den Broeck W., Nevejan N., Muendo P., Van Stappen G. Growth performance and intestinal morphology of African catfish (Clarias gariepinus, Burchell, 1822) larvae fed on live and dry feeds // Aquaculture. 2018. V. 489. P. 70-79. https://doi.org/10.1016/j.aquaculture.2018.01.046.
5. Balogh R. E., Csorbai B., Guti C., Keszte S., Urbányi B., Orbán L., Kovács B. Validation of a male-specific DNA marker confirms XX/XY-type sex determination in several Hungarian strains of African catfish (Clarias gariepinus) // Theriogenology. 2023. V. 205. P. 106-113. https://doi.org/10.1016/j.theriogenology.2023.04.017.
6. Хрусталев Е. И., Курапова T. M., Молчанова K. A., Савина А. В. Темп роста клариевого сома в УЗВ в условиях Калининградской области // Технологии пищевой и перерабатывающей промышленности АПК-продукты здорового питания. 2020. № 2. С. 91-99.
7. Власов В. А. Воспроизводство и выращивание клариевого сома (Clarias gariepinus) в установках с замкнутым водообеспечением (УЗВ) // Рыбоводство и рыбное хозяйство. 2012. № 7. С. 26-35.
8. Muchlisin Z., Nadiah W. N., Nadiya N., Fadli N., Hendri A., Khalil M., Siti-Azizah M. Exploration of natural cryoprotectants for cryopreservation of African catfish, Clarias gariepinus, Burchell 1822 (Pisces: Clariidae) sper-matozoa // Czech Journal of Animal Science. 2015. V. 60. Р. 10-15. DOI:https://doi.org/10.17221/7906-CJAS.
9. Hamid N. H., Daud H. M., Kayansamruaj P., Has-sim H. A., Mohd Yusoff M. S., Abu Bakar S. N., Sris-apoome P. Short- and long-term probiotic effects of Enterococcus hirae isolated from fermented vegetable wastes on the growth, immune responses, and disease resistance of hybrid catfish (Clarias gariepinus × Clarias macrocephalus) // Fish Shellfish Immunol. 2021. V. 114. Р. 1-19. https://doi.org/10.1016/j.fsi.2021.04.012.
10. Boonanuntanasarn S., Sreebun S., Booncherd K., Khaosa-art P., Sooksawat T., Ichida K., Pirarat N., Yazawa R. Cryopreservation of testicular cell in striped catfish (Pangasianodon hypophthalmus) and its effects on apop-tosis, germ-cell specific gene expression and germ cell transplantability // Aquaculture. 2023. V. 570. P. 739370. https://doi.org/10.1016/j.aquaculture.2023.739370.
11. Anil S., Ghafari F., Zampolla T., Rawson D. M., Zhang T. Studies on cryoprotectant toxicity to zebrafsh (Danio rerio) ovarian tissue fragment // CryoLetters. 2010. V. 61. P. 384-385. https://doi.org/10.1016/j.cryobiol.2010.10.080.
12. Omitogun O. G., Ilori O., Olaniyan O., Amupitan P., Oresanya T., Aladele S., Odofin W. Cryopreservation of the Sperm of the African Catfish for the Thriving Aq-uaculture Industry in Nigeria // Current Frontiers in Cryo-preservation. 2012. Р. 305-330. DOI:https://doi.org/10.5772/33490.
13. Steyn G. J., Van Vuren J. H. J., Schoonbe H. J., Chao N. H. Preliminary investigations on the cryopreserva-tion of Clarias gariepinus (Clariidae: Pisces) sperm // Water SA. 1985. N. 11. Р. 15-18.
14. Urbanyi B., Horvath A., Varga Z., Horvath L. Ef-fect of extenders on sperm cryopreservation of African catfish, Clarias gariepinus (Burchell) // Aquaculture Re-search. 2001. V. 30. Р. 145-151. DOI:https://doi.org/10.1046/j.1365-2109.1999.00313.x.
15. Omitogun O. G., Oyeleye O. O., Betiku C. O., Oji-okpota C., Aladele S. E., Sarumi M. B. Potentials of short-term cryopreserved sperm of the giant African catfish, Clarias gariepinus (Burchell, 1822) for aquaculture in Nigeria // Proceedings of the 31st Annual Conference of the Genetic Society of Nigeria. NACGRAB, Moor Plantation, Ibadan, Nigeria (Nov. 6-9, 2006). 2006. Р. 141-146.
16. Богатырева М. М. Оптимизация методов криоконсервации спермы для сохранения генофонда осетровых рыб: дис. ... канд. биол. наук. Астрахань, 2010. 126 с.
17. Пономарева Е. Н., Александрова У. С., Гридина Т. С., Кузов А. А. Особенности развития клариевого сома (Clarias gariepinus, Burchell, 1822) в раннем онтогенезе // Вестн. Астрахан. гос. техн. ун-та. Сер.: Рыбное хозяйство. 2020. № 2. С. 134-141. https://doi.org/10.24143/2073-5529-2020-2-134-141.
18. Chebanov M. S., Galich E. V. FAO Fisheries and Aquaculture Technical Paper. Ankara: FAO, 2013. Р. 558.
19. Polovinkina M. A., Osipova A. D., Osipova V. P., Berberova N. T., Shpakovsky D. B., Gracheva Yu. A., Milaeva E. R. On the Evaluation of the antioxidant activity of water-soluble quaternary ammonium salts containing 2,6-di-tert-butylphenol and pyridine moieties // Russian Chemical Bulletin. 2022. V. 71. N. 10. P. 2218-2223. https://doi.org/10.1007/s11172-016-1362-7.
20. Красильникова А. А., Тихомиров А. М. Корреляция объемов внутриклеточной жидкости сперматозоидов и эндоцеллюлярного протектора в криозащитных средах для осетровых // Естественные науки. 2015. № 3 (52). С. 96-102.
21. Пономарева Е. Н., Красильникова А. А., Тихомиров А. М., Фирсова А. В. Новые биотехнологические методы криоконсервации репродуктивных клеток осетровых видов рыб // Юг России: экология, развитие. 2016. Т. 11. № 1. С. 59-68.
22. Figueroa E., Lee-Estevez M., Valdebenito I., Watanabe I., Oliveira R., Romero J. Effects of cryopreservation on mitochondrial function and sperm quality in fish // Aquaculture. 2019. V. 511. P. 634190. https://doi.org/10.1016/j.aquaculture.2019.06.004.
23. Klaiwattana P., Srisook K., Srisook E., Vuthiphandchai V., Neumvonk J. Effect of cryopreserva-tion on lipid composition and antioxidant enzyme activity of seabass (Lates calcarifer) sperm // Iranian Journal of Fisheries Sciences. 2016. V. 15. P. 157-169.
24. Cabrita E., Martínez-Páramo S., Gavaia P. J., Riesco M. F., Valcarce D. G., Sarasquete C., Herráez M. P., Robles V. Factors enhancing fish sperm quality and emerging tools for sperm analysis // Aquaculture. 2014. V. 432. P. 389-40. https://doi.org/10.1016/j.aquaculture.2014.04.034.
25. Lesaffre E., Feine J., Leroux B., Declerck D. Statistical and Methodological Aspects of Oral Health Re-search. John Wiley & Sons Ltd, Cornwall, 2009. 410 p.