Россия
Россия
Представлены материалы по оптимизации условий выращивания судака в личиночный период развития в искусственно созданной среде. Описаны проблемы, связанные со сложностью процесса эмбрионального и постэмбрионального развития, перехода личинок с эндогенного корма на экзогенный, их выращивания на искусственных кормах, и причины возникновения каннибализма. Поставленная цель заключалась в получении и анализе результатов выращивания личинок судака в условиях замкнутого водообеспечения с использованием в качестве стартовых кормов живых организмов. Приведены результаты исследования воспроизводства судака в индустриальных условиях аквакомплекса Береговой научно-экспедиционной базы «Кагальник» ЮНЦ РАН (Ростовская область, Азовский район), описаны результаты подращивания личинок судака в установке замкнутого водоснабжения. Приведены значения основных гидрохимических показателей среды в рыбоводных емкостях, необходимых для обеспечения нормального роста и развития личинок судака, а также характеристики роста и выживаемости в период экспериментов. В результате разработаны биотехнические нормативы по воспроизводству судака интенсивным методом. Для высокой выживаемости личинок судака необходимо обеспечить своевременное кормление личинок – после заполнения плавательного пузыря и перехода на активное питание; рацион должен быть разнообразным по видовому составу зоопланктона. Для предотвращения каннибализма среди выращиваемых рыб важно поддерживать гидрохимические показатели на оптимальном уровне, а также необходимо наличие кормовых организмов в рыбоводном бассейне.
установка замкнутого водоснабжения, личинки, судак, искусственные корма, живые корма, гидрохимические показатели, каннибализм
Introduction
Commercial aquaculture continues to be a major source of fish products on the world market [1]. Fish is recommended as an important part of a healthy human diet because of its high protein quality, fatty acid composition and micronutrient content [2].
The rapid growth and good nutritive quality of the meat have contributed to an increased interest in aquaculture of perch fish species [3]. Perch (Sander lucioperca Linnaeus, 1758) has been a highly valuable supplementary fish of the pond industry for many years and also a popular sport fishery [4]. For more than two decades, efforts have been made to improve the intensive production of pikeperch in recirculating aquaculture systems (RAS) [5].
Pikeperch breeding is hampered by the difficulties of early ontogenesis, which includes the developmental phase of organogenesis as well as the growth and developmental phase of the immune system [6]. The embryonic period ends with hatching 4-6 days after fertilization. This is followed by the phase of mixotrophic feeding until the yolk sac is completely dissolved. This is followed by gradual adaptation to pelleted feed and transition to the juvenile stage [7]. The three main bottlenecks in pikeperch breeding are the transition from endogenous to exogenous feed, swim bladder bloat and cannibalism. This leads to general problems such as impaired growth and development of the organism and consequently to a high mortality rate [5, 8].
The way to further develop pikeperch production is to optimise growing conditions, both in the larval and fry periods. However, pikeperch larvae are very difficult to raise on artificial feed [9]. The ongoing research to develop a starter artificial diet for pikeperch larvae without the use of natural food, from the early 1960s to date, has failed [10]. The survival rate of pikeperch larvae consuming only artificial food approached 0%, leading to the conclusion that the digestive capacity
of early pikeperch larvae is limited with respect to artificial food (the digestive tract is undeveloped and enzymatic activity is very low) [11].
The best results have been obtained using mixed pikeperch rearing: the first stage in ponds on natural feed and the second stage in pools using mixed feed [12]. Thus, juvenile pikeperch reared on a natural food base can be better prepared as planting material for intensive rearing.
In breeding perch species, intragroup cannibalism is an important factor affecting survival, especially in the early life stages of the fish [13]. The most important factor in the survival of offspring of predatory fish in artificially bred species is the availability of live prey of appropriate size at the beginning of exogenous feeding. The most urgent task for the fish farmer is to ensure the availability of natural food of optimal size in proportion to the size of the larvae of fish that are just beginning to feed [14].
The development of feeding technology for pikeperch larvae when reared in an artificially created environment will make it possible to obtain viable offspring under controlled conditions on a food diet that meets the needs of the organism.
The goal of this research was to carry out experimental work on the rearing of pikeperch larvae (Sander lucioperca Linnaeus, 1758) in RAS on live feed.
Material and research methods
The experimental studies were carried out in the aquatic complex of the Coastal Scientific Expeditionary Base “Kagalnik” of the SSC RAS.
The area of the flume in the RAS was 2.5 m2. The water level in the flume was 0.35 m. Water flow rate (or flow rate) was maintained at 2-4 l/min/m3. The volume of water was 0.9 m3.
Pikeperch larvae were the subject of the study. Larvae for the study were obtained from pikeperch producers from Miusskiy Liman, CJSC which had no deformities, ulcers, injuries, abrasions and reddenings, in the ratio of females to males 1 : 1. The average weight of the producers was 1.77 kg. Reproductive cells for further insemination were obtained using the broodstock method. Insemination was performed by the “dry” method [15]. The eggs were incubated in a pikeperch spawning device [16] on nests with artificial substrate. The percentage of fertilization of the eggs was 70%.
In addition, the pikeperch larvae were fed with natural food organisms by culturing live food (Fig. 1).


a b
Fig. 1. Breeding live food under experimental conditions: a – microalgae; b – Daphnia
A hydrochemical analysis of the water was carried out throughout the pikeperch larval rearing experiment.
Results and discussion
Five days after of the fertilisation of the eggs, the hatching of larvae with an average mass of 0.43 mg was observed. The hatching pikeperch larvae from the spawning unit were transferred to aquaria at the Collective Management Centre of the SSC RAS.
One of the most important rearing parameters is the water temperature. The rearing temperature should be within the thermal comfort zone characteristic of the fish species. The ambient temperature affects the metabolic rate of the fish, which increases with increasing water temperature [17]. That, in its turn, leads to an increased food requirement, a more pronounced larval heterogeneity and, as a consequence in predatory fish species, to potential cannibalism [18]. Cultivation at lower temperatures may have a beneficial effect in slowing cannibalism but is associated with delayed larval growth [19]. The temperature optimum for pikeperch larvae is between 22-24 °C [20]. During the experiment the rearing temperature ranged from 20-22 °C, rarely dropping to
20 °C, more often values close to 22 °C were recorded.
Тable 1
Hydrochemical indicators in the basins of the controlled system when rearing early juvenile zander using live feed
|
Indicator |
Indicator value |
Standards |
|
Water temperature when passing to exogenous feeding, °С |
19-20 |
20-24 (acceptable 18-20) |
|
Water temperature when rearing juveniles to a weight of 0.3 g, °С |
20-22 |
20-24 (acceptable 18-20) |
|
Active medium pH reaction, units |
7.6-8.4 |
6.5-7.5 |
|
Oxygen concentration at the outflow from the system, mg/l |
6.6-7.2 |
6-7 |
|
Total ammonium nitrogen, mg N/l |
0.2 |
no more 2.5 |
|
Nitrites, mg N/l |
0.015 |
no more 0.2 |
The larvae seeded in the tanks were moving vertically and had not yet adopted exogenous nutrition. The larva was not fed while the yolk sac was dissolving.
The attempts to raise pikeperch larvae from the first days of feeding on artificial feed are known to result in 100% mortality, while when using a feed mixture containing 50% natural product (mashed decapsulated Artemia eggs), the survival rate does not exceed 4.3% [22]. According to I. N. Ostroumova [22], only on the 18th day after hatching (length 17 mm), pikeperch develops a stomach and gastric glands (pyloric appendages) and is able to digest incoming food as an adult; therefore, at the initial stage after hatching and before all organs and systems are formed, pikeperch require live food. Therefore, we envisaged the incubation of artemia cysts to produce nauplii as well as the cultivation of other live food organisms such as infusoria, rotifers and microalgae.
During the rearing of the seeding material, fixed daily doses were set at each stage according to earlier recommendations and depending on the size of the fish being reared [23]. At the same time, some artificial feed was added, as the background presence of starter artificial feed allows the larvae to develop a positive response to the odour background of the feed [24]. There were used infusoria and rotifers with slight inclusion of artemia cysts and microalgae as starter live food on day 4 after hatching (larval length 4-5 mm, weight 0.35-0.50 mg). Artemia nauplii were then introduced. Studies have shown that when fed with Artemia salina nauplii, pikeperch larvae have high survival and growth rates, and the composition of fatty acids fully satisfies the fish's need for them [25].
The species composition of the food organisms in pikeperch larval rearing differed depending on the rearing period (Fig. 2).
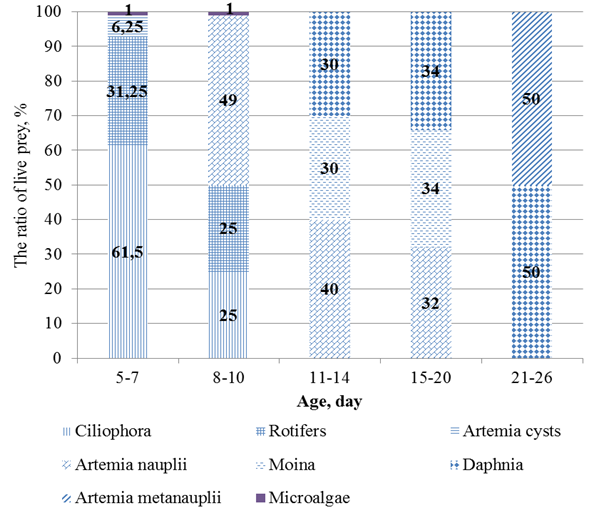
Fig. 2. The ratio of live prey
On the 11th day the larvae were 6.5-7 mm long. At this point, cladoceran crayfish – Moin and juvenile Daphnia were added into the diet. The size of the introduced organisms was up to 275 µm.
On 15th day, the amount of Artemia nauplii (32%) was reduced Moin and Daphnia (34% each) were added to the larval diet (Fig. 3).
After 20 days of hatching the larvae were more than 8 mm in length. The diet during this period contained adult daphnia (50%), up to 900 µm in size, and artemia metanauplius (50%), up to 850 µm in size.
Pikeperch larvae with an initial mass of 0.43 mg after 26 days of rearing with a feeding frequency of 10 times a day reached a maximum mass of 250 mg at
a length of 10 mm. The larval survival rate was 67% and the average daily gain was 7.35 mg/day. The mass accumulation coefficient was 0.58.
Fig. 3. Larva of zander at the age of 15 days
The results of rearing pikeperch larvae after switching to active feeding are presented in Table 2.
Table 2
Fish breeding and biological indicators of pikeperch larvae grown on live feed
|
Indicator |
Value |
|
Weight, mg: Initial; ultimate |
0.43 ± 0.02 191.6 ± 6.6 |
|
Absolute gain, mg |
191.2 |
|
Average daily gain, mg/day |
7.35 |
|
Growing time, days |
26 |
|
Survival rate of fish, % |
67 |
|
Frequency rate of feeding, times/day |
10 |
|
Coefficient of mass accumulation, unit |
0.58 |
During the experiment, no infectious diseases were noticed in the fish. In a single species, abnormalities such as skeletal deformities (5.3%) and lack of swim bladder occupancy were observed. As a result, these larvae did not feed, had a low growth rate and were preyed upon by healthy larvae.
Conclusions
1. In the process of this research the values of the main indicators of the hydrochemical regime were maintained, corresponding to the previously developed and recommended standards, which are necessary for normal growth and development of pikeperch larvae. Characteristics of growth and survival rate of larvae during the period of experiments under these hydrochemical parameters have been determined.
2. The species composition of live food organisms for feeding pikeperch larvae in the regulated conditions depending on the period of rearing is determined.
3. It is important to maintain hydrochemical indicators at an optimal level to prevent negative effects. In addition, a prerequisite of the larval rearing process is the timely removal of dead fish, maintaining the sanitary condition of the fishponds at a high level to avoid secondary contamination of the water.
4. The high survival rates of pikeperch larvae in the experiments conducted are a consequence of a number of factors:
– feeding the larvae was started after they passed the critical stage – filling the swim bladder and their transition to active feeding;
– complex feeding with several types of zooplankton to ensure fullness of diet;
– reducing the likelihood of cannibalism through frequent feeding and availability of food organisms in the fish pool.
1. FAO. The State of World Fisheries and Aquacul-ture 2020. Sustainability in action. Rome, Food and Agri-culture Organization of the United Nations, 2020. 224 p.
2. Schafberg M., Loest K., Müller-Belecke A., Rohn S. Pike-Perch (Sander lucioperca) and Rainbow Trout (Oncorhynchus mykiss) Fed with an Alternative Microor-ganism Mix for Reducing Fish Meal and Oil - Fishes’ Growth Performances and Quality Traits. Foods, 2021, vol. 10, p. 1799. Available at: https://doi.org/10.3390/foods10081799 (accessed: 15.11.2022).
3. Malinovskyi O., Vesely L., Blecha M., Kristan J., Policar T. The substrate selection and spawning behaviour of pikeperch Sander lucioperca L. broodstock under pond conditions. Aquaculture Research, 2018, vol. 49, iss. 11, pp. 3541-3547. Available at: https://doi.org/10.1111/are.13819 (accessed: 15.11.2022).
4. Yanes-Roca C., Holzer A., Mraz J., Vesely L., Malinovskyi O., Policar T. Improvements on Live Feed Enrichments for Pikeperch (Sander lucioperca) Larval Culture. Animals, 2020, vol. 10, p. 401. Available at: https://doi.org/10.3390/ani10030401 (accessed: 17.11.2022).
5. Policar T., Blecha M., Křišťan J., Mráz J., Velíšek J., Stará A., Stejskal V., Malinovskyi O., Svačina P., Sa-marin A. M. Comparison of production efficiency and quality of differently cultured pikeperch (Sander lucioperca L.) juveniles as a valuable product for ongrowing culture. Aquac. Int., 2016, vol. 24, p. 1607-1626. Available at: https://doi.org/10.1007/s10499-016-0050-9 (accessed: 15.11.2022).
6. Schäfer N., Kaya Y., Rebl H. Insights into early ontogenesis: characterization of stress and development key genes of pikeperch (Sander lucioperca) in vivo and in vitro. Fish. Physiol. Biochem., 2021, vol. 47, pp. 515-532. Available at: https://doi.org/10.1007/s10695-021-00929-6 (accessed: 15.11.2022).
7. Güralp H., Pocherniaieva K., Blecha M., Policar T., Pšenička M., Saito T. Development, and effect of water temperature on development rate, of pikeperch Sander lucioperca embryos. Theriogenology, 2017, vol. 104, pp. 94-104. Available at: https://doi.org/10.1016/j.theriogenology.2017.07.050 (ac-cessed: 23.12.2022).
8. Schaefer F. J., Flues S., Meyer S., Peck M. A. Inter- and intra-individual variability in growth and food consumption in pikeperch, Sander lucioperca L., larvae revealed by individual rearing. Aquac. Res., 2017, vol. 48, pp. 800-808. Available at: https://doi.org/10.1111/are.12924 (accessed: 23.12.2022).
9. Liutikov A. A., Korolev A. E. Opyt perevoda molodi sudaka (Sander lucioperca) s estestvennoi pishchi na iskusstvennyi korm [Experience of transferring juveniles of zander (Sander lucioperca) from natural food to artificial food]. Voprosy rybolovstva, 2019, vol. 20, no. 4, pp. 468-481.
10. Liutikov A. A., Korolev A. E., Ostroumova I. N. Kul'tivirovanie rannei molodi sudaka (Sander lucioperca) i okunia (Perca fluviatilis) na iskusstvennykh dietakh [Cultivation of early fry of zander (Sander lucioperca) and perch (Perca fluviatilis) on artificial diets]. Izvestiia KGTU, 2020, no. 56, pp. 34-47.
11. Liutikov A. A., Korolev A. E. Opredelenie opti-mal'noi massy i plotnosti posadki molodi sudaka (Sander lucioperca) pri perevode iz prudov v industrial'nye usloviia [Determining optimal weight and stocking density of juvenile zander (Sander lucioperca) during transfer from ponds to industrial conditions]. Voprosy rybolovstva, 2020, vol. 21, no. 2, pp. 188-202.
12. Müller T., Bódis M., Urbányi B., Bercsényi M. Comparison of the growth of pikeperch Sander lucioperca (L.) and hybrids of pikeperch and Volga pikeperch S. lu-cioperca × S. volgensis (Gmelin, 1789) juveniles reared under controlled conditions. The Israeli Journal of Aquaculture, 2011, vol. 63. Available at: https://doi.org/10.46989/001c.20584 (accessed: 24.12.2022).
13. Król J., Zakęś Z. Effect of dietary L-tryptophan on cannibalism, survival and growth in pikeperch Sander lucioperca (L.) post-larvae. Aquacult. Int., 2016, vol. 24, pp. 441-451. Available at: https://doi.org/10.1007/s10499-015-9936-1 (accessed: 24.12.2022).
14. Csorbai B., Szabó T., Tamás G. H., Kovács É., Béres B., Németh Á., Horváth L. Results and Outcomes of Induced Breeding and Fry Rearing of Zander (Sander lucioperca L.). Turkish Journal of Fisheries and Aquatic Sciences, 2015, vol. 15, pp. 489-493. Available at: http://doi.org/10.4194/1303-2712-v15_2_36 (accessed: 24.12.2022).
15. Nevalennyy A. N., Ponomareva E. N., Sorokina M. N. Biologicheskie osnovy rybovodstva [Biological bases of fish farming]. Moscow, Morkniga Publ., 2016. 434 p.
16. Matishov G. G., Ponomareva E. N., Grigor'ev V. A., Kovalenko M. V., Iakovlev D. A., Sorokina M. N. Nerestovoe ustroistvo dlia sudaka [Spawning device for zander]. Patent 184695 Rossiiskaia Federatsiia, SPK A01K 61/00; no. 2017147098; 06.11.2018.
17. Frisk M., Skov P. V., Steffensen J. F. Thermal opti-mum forpikeperch (Sander lucioperca) and the use of ventilationfrequency as a predictor of metabolic rate. Aquaculture, 2012, vol. 324-325, pp. 151-157. Available at: https://doi.org/10.1016/j.aquaculture.2011.10.024 (ac-cessed: 11.01.2023).
18. Kestemont P., Jourdan S., Houbart M., Mélard C., Paspatis M., Fontaine P., Cuvier A., Kentouri M., Baras E. Size hetero-geneity, cannibalism and competition in cultured predatoryfish larvae: biotic and abiotic influences. Aquaculture, 2003, vol. 227, pp. 333-356. Available at: https://doi.org/10.1016/S0044-8486(03)00513-1 (accessed: 15.11.2022).
19. Zakęś Z. The effect of body size and water temperature on the results of intensive rearing of pike-perch, Stizostedion lucioperca (L.) fry under controlled conditions. Archives of Polish Fisheries, 2012, no. 20 (3). Available at:https://doi.org/10.2478/v10086-012-0020-4 (accessed: 15.11.2022).
20. Mikhailova M. V. Podrashchivanie lichinok sudaka na razlichnykh kormakh [Growth of zander larvae on different kinds of feeds]. Rybnoe khoziaistvo, 1990, no. 5, pp. 58-59.
21. P'ianov D. S. Rybovodno-biologicheskie osoben-nosti vyrashchivaniia tovarnogo sudaka v ustanovkakh zamknutogo vodosnabzheniia. Dissertatsiia … kand. biol. nauk [Fish-breeding and biological features of growing commercial pikeperch in recirculating aquaculture systems. Diss. ... Cand. Biol. Sci.]. Kaliningrad, Izd-vo KGTU, 2016. 142 p.
22. Ostroumova I. N. Biologicheskie osnovy kormleniia ryb [Biological basis of fish feeding]. Saint-Petersburg, Izd-vo GosNIORKh, 2012. 564 p.
23. Makeeva A. P. Embriologiia ryb [Embryology of fish]. Moscow, Izd-vo MGU, 1992. 216 p.
24. Del'mukhametov A. B., P'ianov D. S., Khrustalev E. I. Tekhnologiia vyrashchivaniia sudaka v usloviiakh ustanovok zamknutogo vodosnabzheniia (UZV) [Technology of growing pike perch in conditions of recirculating aquaculture systems (RAS)]. Innovatsionnye tekhnologii v pishchevoi promyshlennosti: nauka, obrazovanie i proizvodstvo: sbornik materialov Mezhdunarodnoi nauchno-tekhnicheskoi konferentsii. Voronezh, Izd-vo VGUIT, 2013. Pp. 632-637.
25. Gamygin E. A., Ponomarev S. V., Belotserkovsky Yu. B., Bolshakova S. G., Mikhailova M. V., Anoshkina E. V., Zharkova G. A. Novoe v kormlenii lichinok sudaka i molodi osetrovykh [New in feeding larvae of pike-perch and juvenile sturgeons]. Sbornik nauchnykh trudov VNIIPRKh. Moscow, 1992. Iss. 67. Pp. 3-4.
















