Russian Federation
Russian Federation
Russian Federation
Russian Federation
Uncontrolled dispersal of species in aquatic ecosystems has become one of the most important environmental problems. The main reason for the acceleration of this process is human economic activity. Aquatic ecosystems are particularly susceptible to attacks by alien species, and the main vector is the discharge of ballast water. Despite the fact that most alien species do not take root, there are species that successfully naturalize in recipient reservoirs. The impact of alien species on aboriginal communities is usually characterized by ambivalence. The Kaliningrad (Vistula) Lagoon, which has 5 ports in its water area, has also been attacked by alien species. The most powerful invasions occurred in 1988 (polychaetes of the genus Marenzelleria), in 1999 (predatory branchial crustaceans Cercopagis pengoi) and in 2010 (bivalves Rangia cuneata).These alien species had a multidirectional impact on food supply of commercial fish species. The first large-scale introduction of Marenzelleria spp. the structure of the bottom community has changed, the role of Chironomidae in the food supply has sharply decreased. The result was a decrease in the catch of Abramis brama, whose favorite food is of Chironomidae. The second large-scale invasion, of predatory Ponto-Caspian cladoceran C. pengoi, changed the structure of the planktonic community of the lagoon. There was a decrease in zooplankton biomass, as a result of which competition for feed resources turned out to be not in favor of the juvenile of Clupea harengus, the main planktophage. The third large-scale invasion of North American bivalve R. cuneata, the most powerful filter, affected the planktonic and bottom communities of the lagoon. The biomass of zooplankton decreased sharply, which created increased trophic conditions among planktophages fish and led to a decrease in catch. At the same time, the phenomenon of Atlantic rangia had a positive effect on other groups of the bottom community, which contributed to an increase in the catch of mollusk-eating fish, the main of which is of Rutilus rutilus.
Kaliningrad (Vistula) Lagoon, biological invasions, planktonic and bottom communities, fish food supply, fish catch
Introduction
The Kaliningrad (Vistula) Lagoon is located in the southeastern part of the Baltic Sea and, due to its location, is subject to intense anthropogenic impact; there are 5 ports in its waters. One of the results of economic activities consists in biological invasions. Most invasive species do not take root, but some of them cause large-scale environmental consequences and significant economic damage [1].
Aquatic invasions can affect biodiversity and the ecosystem at various levels, for example, by displacing native species, disrupting trophic relationships and changing nutrient flows [2-5]. Alien species enter water bodies in various ways. The main vectors of their spread are natural dispersal, intentional and unintentional acclimatisation, ballast water of ships etc. [6].
The Vistula Lagoon has also been subjected to “attacks” by alien species, the most widespread of which were immigration into the planktonic community, Cercopagis (Cercopagis) pengoi (Ostroumov, 1891) in 1999 [7] and benthic – polychaetes of the genus Marenzelleria Mesnil, 1896 (M. neglecta Sikorski and Bick, 2004 and M. viridis (Verrill, 1873)) in 1988 and Rangia cuneata (G. B. Sowerby I, 1832) in 2010. The main vector of penetration of alien species into the planktonic and bottom communities of the lagoon is the ballast water of ships [8]. Under the current conditions, the stocks of most species of aquatic biological resources remain relatively stable, the dynamics of their numbers and biomass are mainly determined by natural causes (spawning conditions, development in the first year of life, food supply), which in turn allows for stable extraction of aquatic biological resources [9, 10].
The study was aimed at assessing the impact of invasive species that have penetrated the planktonic and benthic communities of the Kaliningrad (Vistula) Lagoon on the food supply and fish catch.
Material and methods
The materials were zooplankton and zoobenthos samples collected in the Kaliningrad (Vistula) Lagoon from 1980 to 2020. Samples were taken once a month, from April to November, at nine standard stations
(Fig. 1), the location of the stations corresponded to the hydrological division of the lagoon [11]. Zooplankton samples were integrally collected with a Dyachenko-Kozhevnikov planktobathometer with a volume of 5 l from three horizons (surface 0.5 m, middle 1.5 m and lower 2.5 m) and fixed with 4% formalin with sucrose. Further processing was performed in the laboratory with the accepted method [12]. The biomass of zooplankton organisms was determined from the dependence of body weight on the length of an individual with the programme of E. V. Shchukina was used.
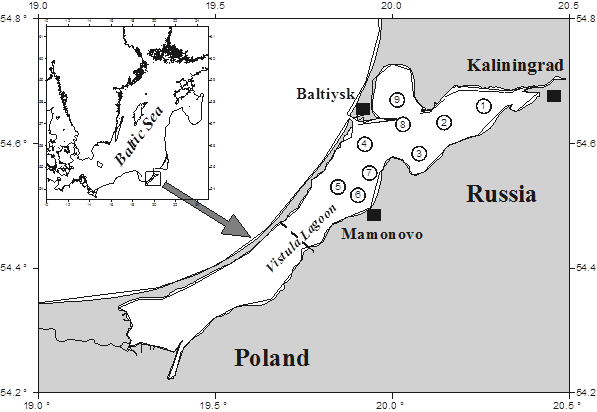
Fig. 1. Study area and location of stations in the Kaliningrad (Vistula) Lagoon
Zoobenthos was collected by a Petersen grab with a capture area of 0.025 m2. Samples were washed in a benthic bag having 0.5 mm mesh size and fixed with 4% formalin. Further processing was performed in the laboratory with the accepted method [13]. The synonyms of identified taxa are given in accordance with the World Register of Marine Species [14].
Statistical data on the catch of aquatic biological resources in the Kaliningrad (Vistula) Lagoon are provided by the West Baltic Territorial Administration of the Federal Agency for Fisheries. The data were ranked into four periods: the first one – 1980-1987 – before the introduction of alien species, the second one – 1988-1998 – the introduction and influence of polychaetes of the genus Marenzelleria, the third one – 1999-2009 – the introduction and influence of the cladoceran Cercopagis (Cercopagis) pengoi and the fourth one – 2010-2020 – the introduction and influence of the bivalves Rangia cuneata. Since the data series did not have a normal distribution and homogeneity of variances, the Kruskal-Wallis test (H) was used. To determine the relationship between the biomasses of individual groups of zooplankton, zoobenthos and the catch of the main commercial fishes, Spearman’s rank correlation was calculated. In all analyses, the significance level was set at p = 0.05. Mean values (M) and standard error of the mean (± SE) were calculated. For descriptive statistics, Spearman’s rank correlation and Kruskal-Wallis test, the statistical package Statistica version 6.0 was used.
Research results
Zooplankton. The statistically significant differences in the biomass of Copepoda were not found between the study periods (H = 5.263, p = 0.3846). The biomass varied from 729.87 ± 306.55 to 871.18 ± ± 241.61 mg/m3 (Fig. 2, a).


Fig. 2. Biomass dynamics of the main zooplankton groups (M ± SE)
in the Kaliningrad (Vistula) Lagoon in 1980-2020: a – Copepoda; b – Cladocera
|
c
|
Fig. 2 (ending). Biomass dynamics of the main zooplankton groups (M ± SE)
in the Kaliningrad (Vistula) Lagoon in 1980-2020: c – Rotifera
For Cladocera statistically significant differences in biomass were noted between the studied periods (H = 9.141, p = 0.0275). Minimum biomasses of Cladocera were recorded in the period before the introduction of C. pengoi in 1980-1987 (197.21 ± 136.76 mg/m3) and 1988-1998 (207.06 ± 69.55 mg/m3). In 1999-2009 biomass sharply increased and averaged 526.41 ± 102.43 mg/m3. After the introduction of the R. cuneata, the biomass of Cladocera decreased to 322.38 ± 108.84 mg/m3 in 2010-2020 (Fig. 2, b). The statistically significant differences in the biomass of Rotifera between the periods studied were not found (H = 5.314,
p = 0.1502). The maximum biomass was recorded in 1980-1987 (108.02 ± 30.83 mg/m3). Then the biomass decreased and the minimum values were recorded after the introduction of C. pengoi in 1999-2009
(48.30 ± 10.21 mg/m3) and R. cuneata in 2010-2020 (44.16 ± 21.29 mg/m3) (Fig. 2, c).
Zoobenthos. For Chironomidae statistically significant differences in biomass were recorded between the studied periods (H = 12.667, p = 0.0054). Before the appearance of the invasive species in 1980-1987, the biomass of Chironomidae was 13.53 ± 1.61 g/m2. After the introduction of polychaetes of the genus Marenzelleria in 1988-1998, minimal biomasses were recorded 5.97 ± 1.37 g/m2. Then the biomass gradually increased and its maximum reached after the introduction of R. cuneata in 2010-2020 (23.52 ± 5.16 g/m2) (Fig. 3, a).




Fig. 3. Biomass dynamics of the main zoobenthos groups (M ± SE) in the Kaliningrad (Vistula) Lagoon in 1980-2020:
a – Chironomidae; b – Oligochaeta; c – Polychaeta; d – Bivalvia
The statistically significant differences in the biomass of Oligochaeta were not found between the studied periods (H = 5.535, p = 0.1366). The biomass varied from 1.74 ± 0.49 to 3.80 ± 0.84 g/m2 (Fig. 3, b).
For Polychaeta statistically significant differences in biomass were noted between the studied periods (H = 21.054, p = 0.0001). Minimum biomasses were recorded in the period before the introduction
of alien species in 1980-1987 (1.96 ± 0.55 g/m2). In 1988-1998, the biomass of Polychaeta sharply increased to maximum values (8.76 ± 2.14 g/m2). Their biomass decreased and averaged 2.24 ± 0.37 g/m2
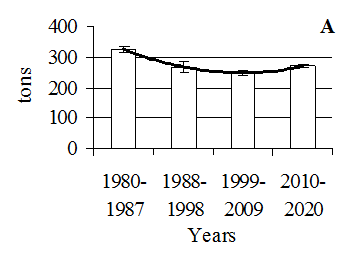
in 1999-2009. After the introduction of R. cuneata, biomass began to increase again and in 2010-2020 reached the values 6.52 ± 0.77 g/m2 (Fig. 3, c). The statistically significant differences in the biomass of Bivalvia between the studied periods were found (H = 26.809, p = 0.00001). In 1980-1987, the biomass was 16.12 ± 6.55 g/m2. In 1988-2009, their biomass was at a low level (in 1988-1998 – 4.78 ± 0.76 g/m2 and in 1999-2009 – 3.27 ± 0.86 g/m2). Since 2010, after the introduction of R. cuneata, the biomass of Bivalvia has sharply increased to 747.92 ± ± 152.00 g/m2 (Fig. 3, d). Catch of commercial fishes. For bream Abramis brama (Linnaeus, 1758) statistically significant differences in catch levels between the periods studied were established (H = 15.221, p = 0.0016). The maximum catch of bream was recorded in 1980-1987 (325.1 ± 10.1 t/year). Then it decreased and was at its minimum in 1999-2009 after the introduction of C. pengoi (248.3 ± 9.1 t/year) (Fig. 4, a).
 |
|
|
|
|
|
|
|
Fig. 4. Catch dynamics of the commercial fishes (M ± SE) in the Kaliningrad (Vistula) Lagoon in 1980-2020:
a – bream Abramis brama; b – pike-perch Sander lucioperca;
c – sabrefish Pelecus cultratus; d – Baltic herring Clupea harengus; e – roach Rutilus rutilus
For pike-perch Sander lucioperca (Linnaeus, 1758) statistically significant differences in catch levels were noted between the study periods (H = 12.607, p = 0.0056). The pike-perch catch level has been steadily declining from 208.7 ± 9.1 t/year in 1980-1987 to 133.8 ± 2.7 t/year in 2010-2020 (Fig. 4, b).
Statistically significant differences in catch levels of sabrefish Pelecus cultratus (Linnaeus, 1758) were recorded between the periods under study (H = 29.012, p = 0.000002). The minimum level of sabrefish catch was recorded in 1980-1987 (13.1 ± 6.6 t/year). Then it increased sharply and reached its maximum during the period of the introduction of C. pengoi in 1999-2009 (76.9 ± 3.3 t/year). After the introduction of R. cuneata, it dropped slightly to 61.6 ± 4.3 t/year in 2010-2020 (Fig. 4, c). For the Baltic herring Clupea harengus Linnaeus, 1758, statistically significant differences in the catch levels were found between the study periods (H = 20.295, p = 0.0001). The maximum catch level was in 1980-1987 (7,192.5 ± 300.6 t/year). Then the catch level decreased, and the minimum values were recorded after the introduction of C. pengoi in 1999-2009 (2,043.5 ± 68.1 t/year). Since 2010, the catch level of Baltic herring removal has slightly increased to 2,522.7 ± 171.3 t/year in 2010-2020 (Fig. 4, d).
For roach Rutilus rutilus (Linnaeus, 1758), statistically significant differences in catch rates were recorded between the study periods (H = 30.666, p = 0.000001). In the initial study period of 1980-1987, the roach catch rate was 28.7 ± 3.0 t/year. After the introduction of Merenzelleria spp. in 1988-1998, the minimum catch rate was 18.3 ± 2.6 t/year. Then it gradually increased to a maximum in 2010-2020, 81.6 ± 2.3 t/year (Fig. 4, e).
Correlations between biomasses of the main groups of zooplankton, zoobenthos and catch of the main commercial fishes. With Spearman’s rank correlation, shown were statistically significant correlations between the biomasses of the main groups of zooplankton, zoobenthos and the catch of commercial fishery species (bream, pike-perch, sabrefish, Baltic herring and roach) in the Vistula Lagoon (Table).
Correlation relationships between the biomasses of the main groups of zooplankton
(Copepoda, Cladocera, Rotifera), zoobenthos (Chironomidae, Oligochaeta, Polychaeta and Bivalvia)
and the catch of the main commercial fishes (bream, pike-perch, sabrefish, Baltic herring and roach)
in the Kaliningrad (Vistula) Lagoon in 1980-2020 (Spearman’s rank correlation, relationships at the level
of statistical significance p < 0.05 are highlighted in bold)
|
Parameter |
Copepoda |
Cladocera |
Rotifera |
Chironomidae |
Oligochaeta |
Polychaeta |
Bivalvia |
Bream |
Pike-perch |
Sabrefish |
Baltic herring |
|
Cladocera |
0.20 |
– |
|
|
|
|
|
|
|
|
|
|
Rotifera |
0.36 |
0.17 |
– |
|
|
|
|
|
|
|
|
|
Chironomidae |
–0.34 |
0.17 |
–0.05 |
– |
|
|
|
|
|
|
|
|
Oligochaeta |
–0.21 |
0.06 |
0.22 |
0.36 |
– |
|
|
|
|
|
|
|
Polychaeta |
–0.12 |
–0.28 |
–0.41 |
–0.21 |
–0.30 |
– |
|
|
|
|
|
|
Bivalvia |
–0.43 |
–0.20 |
–0.25 |
0.30 |
0.10 |
0.42 |
– |
|
|
|
|
|
Bream |
–0.02 |
–0.37 |
0.21 |
–0.04 |
0.29 |
–0.01 |
0.35 |
– |
|
|
|
|
Pike-perch |
0.13 |
–0.38 |
0.29 |
–0.11 |
0.33 |
–0.12 |
0.05 |
0.86 |
– |
|
|
|
Sabrefish |
0.08 |
0.41 |
–0.13 |
0.21 |
0.04 |
–0.06 |
–0.15 |
–0.46 |
–0.44 |
– |
|
|
Baltic herring |
–0.12 |
–0.46 |
0.11 |
–0.02 |
0.16 |
0.04 |
0.25 |
0.75 |
0.68 |
–0.61 |
– |
|
Roach |
–0.26 |
0.20 |
–0.25 |
0.55 |
0.33 |
–0.03 |
0.36 |
–0.11 |
–0.22 |
0.62 |
–0.28 |
In zooplankton, recorded were statistically significant positive correlations only between the biomasses of Copepoda and Rotifera; and in zoobenthos, between Chironomidae and Oligochaeta, Polychaeta and Bivalvia. Based on the catch of commercial fishes in the Vistula Lagoon, established were positive correlations between bream, pike-perch and Baltic herring; and negative ones, these three species with the catch
of sabrefish. A positive correlation between the catch of sabrefish and roach was found (Table).
Negative statistically significant correlations were recorded between the biomasses of zooplankton and zoobenthos groups: Copepoda and Chironomidae, Copepoda and Bivalvia, Rotifera and Polychaeta.
The catch of bream, pike-perch and herring had negative correlations with the Cladocera biomass, while the catch of sabrefish and the Cladocera biomass correlated positively. The catch of roach had positive statistically significant correlations with the biomass of Chironomidae, Oligochaeta and Bivalvia. Positive correlations were also recorded between the Bivalvia biomass and bream catch (Table).
Discussion
In 1980-1987, before the most significant invasive species appeared in the planktonic and benthic communities of the Vistula Lagoon, the catch of bream, pike-perch and Baltic herring was at its maximum (Fig. 4, a, b, d). The catch of sabrefish and roach was low (Fig. 4, c, e). At the same time, the biomass of Copepoda, Rotifera, Chironomidae, Oligochaeta and Bivalvia was high (Fig. 2, a, c, 3, a, b, c), while the biomass of Cladocera and Polychaeta was low (Fig. 2, b, 3, c). The main benthophages in the Vistula Lagoon are bream, eel Anguilla anguilla (Linnaeus, 1758), roach, white bream Blicca bjoerkna (Linnaeus, 1758), vimba bream Vimba vimba (Linnaeus, 1758) and ruffe Gymnocephalus cernua (Linnaeus, 1758); such predators as pike-perch, sabrefish and perch Perca fluviatilis Linnaeus, 1758 [15].
The first large-scale introduction of polychaetes of the genus Marenzelleria into the benthic community occurred in 1988; the following year, the species formed a population and spread throughout the entire water area of the lagoon. This expansion resulted in a sharp change in the existing structure of the benthic community [8]. A significant decrease in the biomass of Copepoda, Rotifera, Chironomidae, Oligochaeta, and Bivalvia was recorded in 1988-1998 (Fig. 2, a, c, 3, a, b, c). The biomass of Polychaeta increased by 4.5 times, with Marenzelleria spp. dominating (Fig. 3, c). During this period, a decrease in the catch of bream, pike-perch, Baltic herring and roach was recorded (Fig. 4, a, b, d, e) and an increase in the catch of sabrefish (Fig. 4, c). A feature of the biology of Marenzelleria spp. is that they build deep burrows (up to
25-35 cm). By making respiratory movements above the surface of the ground, they create extensive “moving” areas on silty ground [16, 17]. Probably, the larvae of Chironomidae cannot settle to the bottom because of this and die. In the Baltic Sea basin, Marenzelleria spp. have been recorded in the diet of bream, roach, perch, pike-perch, ruffe, carp Cyprinus carpio Linnaeus, 1758, eel, pike Esox lucius Linnaeus, 1758, Baltic herring, European flounder Platichthys flesus (Linnaeus, 1758), sculpin Myoxocephalus scorpius (Linnaeus, 1758), lumpfish Cyclopterus lumpus Linnaeus, 1758, three-spined stickleback Gasterosteus aculeatus Linnaeus, 1758, nine-spined stickleback Pungitius pungitius (Linnaeus, 1758), gobies of the genus Pomatoschistus Gill, 1863 and round goby Neogobius melanostomus (Pallas, 1814) [18-21]. Bream is the most commercially important of the benthophages of the lagoon. Due to the structure of its mouth apparatus, the bream poorly utilises Marenzelleria spp. in its diet [18, 19], which is probably why the food supply for the bream worsened, and it began to leave the lagoon for the coastal part of the Baltic Sea in summer to feed [15]. It is possible that Marenzelleria spp. had the same negative effect on other benthophage fishes. Just as in the Curonian Lagoon, a change in the spatial distribution of pike-perch in the Vistula Lagoon was recorded at that time, which occurred due to deterioration in food supply. This led to the pike-perch moving from the open part of the lagoon to the shallows areas of the lagoon, where it fed on young Cyprinidae fish and previously unrecorded sabrefish, as well as to the coastal part of the Baltic Sea, where dense accumulations of Baltic herring and sprat Sprattus sprattus (Linnaeus, 1758) are created [9, 10].
The second large-scale introduction of an alien species into the ecosystem occurred in 1999. The predatory cladoceran species of Ponto-Caspian origin C. pengoi was first recorded in the lagoon zooplankton [7]. Its introduction resulted in a restructuring of the zooplankton community. Zooplankton in the Vistula Lagoon is represented mainly by Copepoda, Cladocera and Rotifera. Before the introduction of C. pengoi, the proportion of Copepoda in the biomass of the Vistula Lagoon zooplankton was about 70%. However, the introduction and naturalisation of a large predator into the planktonic community (1999-2009) contributed to a decrease in the role of Copepoda in the biomass in some years by up to 30%, while the share of Rotifera increased to 44%. Overall, a significant decrease in the biomass of Rotifera, Polychaeta, and Bivalvia was recorded in 1999-2009 (Fig. 2, c, 3, c, d). The biomass of Cladocera increased by 2.5 times, with C. pengoi dominating (Fig. 2, b). During this time, a decrease in the catch of bream, pike-perch, and Baltic herring was noted
(Fig. 4, a, b, d), while the catch of sabrefish and roach increased (Fig. 4, c, e). Although the biomass of Copepoda has not changed, in the main food species Eurytemora affinis affinis (Poppe, 1880) and Acartia (Acanthacartia) tonsa Dana, 1849, nauplia and juvenile copepodite stages began to predominate in summer, which was reflected in a decrease in the biomass of the food base of planktivorous fish.
The main planktivorous fish in the lagoon are juvenile Baltic herring, as well as smelt Osmerus eperlanus (Linnaeus, 1758), but its numbers have been extremely small since the early 1990s. Larvae and juveniles of many fish species also feed on zooplankton. It was found that C. pengoi is a competitor to planktivorous fishes, since it feeds on zooplankton, among other things [22]. The issue of planktophages being fed C. pengoi of the Vistula Lagoon has not been sufficiently studied. There is evidence that Baltic herring, smelt, white fish Coregonus albula (Linnaeus, 1758), bleak Alburnus alburnus (Linnaeus, 1758), roach, perch and bream consume C. pengoi [23]. Tail spines of C. pengoi have been found in the intestines of juvenile Baltic herring. The presence of a large number of tail spines in the intestines (up to 10-15 pieces) may indicate that they are not evacuated from the intestines, which can lead to the death of juvenile and a decrease in the spawning part of the population of spring-spawning Baltic herring, for which the Vistula Lagoon is the main spawning ground [24], it is possible that the same can be recorded in commercial fishes at the stage of their feeding on zooplankton. Sabrefish mainly selectively feeds on Cladocera (Leptodora kindtii (Focke, 1844), Bosmina (Eubosmina) coregoni Baird, 1857, Daphnia longispina (O. F. Müller, 1776)) and the mass development of the new invasive species C. pengoi has significantly improved its food supply and the survival of its juvenile. On the other hand, an analysis of the sabrefish diet made it clear that large individuals are facultative predators that, in addition to invertebrates, feed on young smelt, ruffe, pike-perch and other fish species. Thus, sabrefish, through the “predator-prey” mechanism, can influence the size of the stocks of other commercial fishes. In 2010, the third large-scale introduction of North American bivalve R. cuneata into the benthic community occurred [8]. The species can be characterised by high filtration activity, has become an important component of the lagoon ecosystem and plays a significant role in self-purification and clarification of water [25]. In 2010-2020, the biomass of Cladocera and sabrefish decreased (Fig. 2, b, 4, c). The biomass of all zoobenthos groups (Fig. 3) and the catch of bream, Baltic herring and roach increased (Fig. 4, a, d, e). The biomass of Bivalvia in the open part of the lagoon increased by more than two orders of magnitude (Fig. 3, d). The recorded decrease in the biomass of Cladocera had a negative impact on the food supply of planktophagous fish, especially sabrefish. In turn, the decrease in the catch of sabrefish had a positive effect on the catch of bream, herring and roach. According to the feeding type, R. cuneata belongs to random filter feeders. They feed on large amounts of detritus and phytoplankton, as a result of which they can compete for food resources with native species of zooplankton. On the other hand, R. cuneata is an important food item in estuarine ecosystems for fish, crabs, gastropods, waterfowl and ctenophores [8, 25, 26]. In the Kaliningrad (Vistula) Lagoon, R. cuneata is used in the diet
of roach, white bream, eel, silver carp Carassius gibelio (Bloch, 1782) and European flounder [26], which significantly improved the food supply of some benthophagous fish that feed on mollusks.
Conclusion
Large-scale introductions of alien invertebrates into the planktonic and benthic communities of the Kaliningrad (Vistula) Lagoon, that have occurred over the past 40 years, have had a multidirectional impact on the food supply of native fish species. The introduction of North American species of the genus Marenzelleria into the benthic community has resulted in a deterioration in the food supply of benthophagous fish, including bream, the main commercial target, and a decrease in their catch. Adaptation of the alien Ponto-Caspian cladoceran Cercopagis (Cercopagis) pengoi in the planktonic community has significantly affected the food supply of planktophagous fish and especially juvenile Baltic herring, for which the Lagoon is the main spawning ground. As a result, a gradual decrease in the catch of the spawning part of the Baltic herring population in the Lagoon has begun. The third large-scale introduction into the Lagoon occurred in the benthic community. The North American bivalve Rangia cuneata, had a negative impact on the planktonic community, but, on the other hand, a positive impact on the benthic community, which contributed to the restoration of the food supply of benthophagous fish. The use of roach in the diet of R. cuneata has affected the increase in the catch of the species. No restoration of the catch of other benthophagous fish (including bream) is currently recorded.
Thus, the introduction of alien species into the ecosystem of the Kaliningrad (Vistula) Lagoon had a generally negative impact on the food supply of commercial fishes, which contributed to a decrease in the catch of valuable commercial fish species. In order to protect the water body from subsequent introductions of alien species, it is recommended to disinfect the ballast water of ships in ports.
1. Pimentel D., Zuniga R., Morrison D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecological Economics, 2005, vol. 52, no. 3, pp. 273-288. https://doi.org/10.1016/j.ecolecon.2004.10.002.
2. Havel J. E., Kovalenko K. E., Thomaz S. M., Amalfitano S., Kats L. B. Aquatic invasive species: challenges for the future. Hydrobiologia, 2015, vol. 750, no. 1, pp. 147-170. https://doi.org/10.1007/s10750-014-2166-0.
3. Bellard C., Cassey P., Blackburn T. M. Alien species as a driver of recent extinctions. Biology Letters, 2016, vol. 12, no. 2, 20150623. https://doi.org/10.1098/rsbl.2015.0623.
4. Cuthbert R. N., Pattison Z., Taylor N. G., Verbrugge L., Diagne C., Ahmed D. A., Leroy B., Angulo E., Briski E., Capinha C., Catford J. A., Dalu T., Essl F., Gozlan R. E., Haubrock P. J., Kourantidou M., Kramer A. M., Renault D., Wasserman R. J., Courchamp F. Global economic costs of aquatic invasive alien species. Science of The Total Environment, 2021, vol. 775, p. 145238. https://doi.org/10.1016/j.scitotenv.2021.145238.
5. Telesh I. V., Naumenko E. N. Ambivalence of planktonic invaders and transformation of communities. Inland Water Biology, 2024, vol. 17, no. 1, pp. 188-196. https://doi.org/10.1134/S1995082924010164.
6. Biologicheskie invazii v vodnykh i nazemnykh ekosistemakh [Biological invasions in aquatic and terrestrial ecosystems]. Pod redaktsiei A. F. Alimova, N. G. Bogutskoi. Moscow, Tovarishchestvo nauchnykh izdanii KMK, 2004. 436 p.
7. Naumenko E. N., Polunina Iu. Iu. Cercopagis pengoi (Ostroumov, 1891) (Crustacea: Cladocera) – novyi vselenets v Vislinskii zaliv Baltiiskogo moria [Cercopagis pengoi (Ostroumov, 1891) (Crustacea: Cladocera) – new inhabitant of the Vistula Lagoon of the Baltic Sea]. Vidy-vselentsy v evropeiskikh moriakh Rossii. Apatity, 2000. Pp. 121-129.
8. Rudinskaya L. V., Gusev A. A. Invasion of the North American wedge clam Rangia cuneata (G. B. Sowerby I, 1831) (Bivalvia: Mactridae) in the Vistula Lagoon of the Baltic Sea. Russian Journal of Biological Invasions, 2012, vol. 3, no. 3, pp. 220-229. DOI:https://doi.org/10.1134/S2075111712030071.
9. Bandurin K. V., Amosova V. M., Golubkova T. A., Arkhipov A. G. Sostoianie i ekspluatatsiia zapasov promyslovykh vidov ryb rossiiskoi chasti Baltiiskogo moria, Kurshskogo, Kaliningradskogo i Finskogo zalivov [Status and exploitation of stocks of commercial fish species in the Russian part of the Baltic Sea, the Curonian, Kaliningrad and Finnish Bays]. Trudy VNIRO, 2024, vol. 195, pp. 24-34. https://doi.org/10.36038/2307-3497-2024-195-24-34.
10. Khlopnikov M. M., Nazarov N. A., Golubkova T. A. Issledovaniia v Baltiiskom more i ego zalivakh [Research in the Baltic Sea and its bays]. Voprosy rybolovstva, 2009, vol. 10, no. 4 (40), pp. 656-666.
11. Chechko V. A. Analysis of space and time variations in the suspended matter distribution in Kaliningrad Bay of the Baltic Sea. Water Resources, 2002, vol. 29, no. 4, pp. 388-395. https://doi.org/10.1023/A:1019670223032.
12. Metodicheskie rekomendatsii po sboru i obrabotke materialov pri gidrobiologicheskikh issledovaniiakh na presnykh vodoemakh. Zooplankton i ego produktsiia [Methodological recommendations for the collection and processing of materials during hydrobiological studies in freshwater reservoirs. Zooplankton and its products]. Leningrad, Izd-vo GosNIIORKh, 1984. 33 p.
13. Metodicheskie rekomendatsii po sboru i obrabotke materialov pri gidrobiologicheskikh issledovaniiakh na presnykh vodoemakh. Zoobentos i ego produktsiia [Methodological recommendations for the collection and processing of materials during hydrobiological studies in freshwater reservoirs. Zoobenthos and its products]. Leningrad, Izd-vo GosNIIORKh, 1983. 51 p.
14. WoRMS Editorial Board. World Register of Marine Species. Available at: https://www.marinespecies.org. at VLIZ. 2024 (accessed: 28.05.2024). https://doi.org/10.14284/170.
15. Naumenko E. N., Khlopnikov M. M., Rudinskaia L. V. Potoki energii v ekosisteme Vislinskogo (Kaliningradskogo) zaliva Baltiiskogo moria [Energy flows in the ecosystem of the Vistula (Kaliningrad) Lagoon of the Baltic Sea]. Zhurnal Sibirskogo federal'nogo universiteta. Biologiia, 2012, vol. 5, no. 2, pp. 184-202.
16. Renz J. R., Forster S. Are similar worms different? A comparative tracer study on bioturbation in the three sibling species Marenzelleria arctia, M. viridis, and M. neglecta from the Baltic Sea. Limnology and Oceanography, 2013, vol. 58, no. 6, pp. 2046-2058. https://doi.org/10.4319/lo.2013.58.6.2046.
17. Kocheshkova O. V., Ezhova E. E. Polychaetes of Marenzelleria genus (Spionida) in the Southeastern Baltic Sea (Russian EEZ). Russian Journal of Biological Invasions, 2018, vol. 9, no. 3, pp. 219-227. https://doi.org/10.1134/S2075111718030050.
18. Debus L. Veränderungen der Ernährung benthophager Fische (Blei, Karpfen und Kaulbarsch) als Resultat eines grossräumigen Besatzexperimentes mit Karpfen. Fischerei-Forschung, Rostock, 1991, vol. 29, no. 4, pp. 66-71.
19. Golubkov S., Tiunov A., Golubkov M. Food-web modification in the eastern Gulf of Finland after invasion of Marenzelleria arctia (Spionidae, Polychaeta). NeoBiota, 2021, vol. 66, pp. 75-94. https://doi.org/10.3897/neobiota.66.63847.
20. Guschin A. V., Ezhova E. E., Borovikova E. A. Feeding of the alien round goby Neogobius melanostomus (Perciformes: Gobiidae) in the Southeastern Baltic. Russian Journal of Biological Invasions, 2022, vol. 13, no. 1, pp. 32-40. https://doi.org/10.1134/S2075111722010052.
21. Winkler H. M., Debus L. Is the polychaete Marenzelleria viridis an important food item for fish? Proceedings of the 13th Symposium of the Baltic Marine Biologists, 1996, pp. 147-151.
22. Lehtiniemi M., Gorokhova E. Predation of the introduced cladoceran Cercopagis pengoi on the native copepod Eurytemora affinis in the northern Baltic Sea. Marine Ecology Progress Series, 2008, vol. 362, pp. 193-200. https://doi.org/10.3354/meps07441.
23. Lehtiniemi M., Lindén E. Cercopagis pengoi and Mysis spp. alter their feeding rate and prey selection under predation risk of herring (Clupea harengus membras). Marine Biology, 2006, vol. 149, no. 4, pp. 845-854. https://doi.org/10.1007/s00227-006-0243-2.
24. Naumenko E. N., Ushakova A. Iu. Spektry pitaniia molodi ryb Vislinskogo zaliva Baltiiskogo moria [Feeding spectra of juvenile fish of the Vistula Lagoon of the Baltic Sea]. Izvestiia KGTU, 2018, no. 51, pp. 13-24.
25. Gusev A. A., Feneva I. Iu. Rangia cuneata (G. B. Sowerby I, 1832). Samye opasnye invazionnye vidy Rossii (TOP – 100) [Rangia cuneata (G. B. Sowerby I, 1832). The most dangerous invasive species in Russia (TOP 100)]. Moscow, Tovarishchestvo nauchnykh izdanii KMK, 2018. Pp. 343-351.
26. Kornijów R., Pawlikowski K., Drgas A., Rolbiecki L., Rychter A. Mortality of post-settlement clams Rangia cuneata (Mactridae, Bivalvia) at an early stage of invasion in the Vistula Lagoon (South Baltic) due to biotic and abiotic factors. Hydrobiologia, 2018, vol. 811, no. 1, pp. 207-219. https://doi.org/10.1007/s10750-017-3489-4.





















