Russian Federation
Regulation of the growth rate of Phaeodactylum tricornutum opens up prospects for the organization of efficient industrial production of live feed biomass. We studied the effect of two plant hormones, indole-3-butyric acid (3-IBA) and indole-3-acetic acid (3-IAA), on the growth and biochemical composition of Ph. tricornutum in an enrichment culture. The effective 3-IBA concentration of 0.2 ∙ 10–5 M increased the density of the culture by 373% on day 14of cultivation compared to the control. High concentrations of 3-IAA either inhibited the development of the culture or did not have a significant effect on its growth. A stimulating effect of exposure to 3-IAA at 0.1 ∙ 10–5 M on the growth was recorded on day 12 of cultivation and amounted to 328.2% compared to the control. A stimulating effect of 3-IBA exposure on protein accumulation was recorded on day 4 of cultivation and amounted to 27.9% compared to the control. A stimulating effect of 3-IAA on protein synthesis was recorded on day 12 of cultivation of Ph. tricornutum. The protein concentration during this period was 1.2-fold higher than in the control. Indole-3-butyric acid did not have any effect on lipid accumulation, compared to the control, throughout the cultivation time. On day 14 of cultivation under the exposure to 3-IAA, the concentration of lipids was 1.5-fold higher than in the control and in the group exposed to 3-IBA. Indole-3-butyric acid did not show any effect on the accumulation of carbohydrates in the Ph. tricornutum culture throughout the cultivation period. On day 4 of cultivation, the carbohydrate concentration in the culture exposed to 3-IAA increased by 240% compared to the control. At the end of the experiment (14 days), no significant differences between the experi-mental cultures in the carbohydrate concentration were observed. For 8 days of Ph. tricornutum cultivation, the chlorophyll concentration increased by 927.4 and 1 178.6% as a result of exposure to 3-IBA and 3-IAA, respec-tively.
plant hormones, microalgae, auxins, indole-3-butyric acid, indole-3-acetic acid, aquaculture, Phaeodactylum tricornutum
Introduction
Cultured microalgae are an integral part of the hatchery production of many farmed fish, shellfish and other commercially important aquaculture species. There is an extensive published literature on the use of various microalgae strains as a source of bioactive components, cultivation techniques, concentration, and practical applications. In the last decade, aquaculture production of bivalve culture has increased by 2.7% per year on an annualized basis, while production of carnivorous fish species has increased by 8.4% annually [1].
Aquaculture enterprises producing juvenile finfish and molluscs represent the most numerous microalgae farms worldwide. Along with these, there is a trend towards the use of microalgae as a micro-feed ingredient in formulated feeds for terrestrial animals.
The cultivation of bivalves does not require the use of artificial feeds. However, shellfish cultivation is highly dependent on the supply of live microalgae [2]. All developmental stages of bivalves are directly dependent on microalgae as feed. Therefore, in bivalve farming, a number of microalgae strains are cultured for mother stock rearing, conditioning, larval rearing and spat feeding.
The need for live microalgae during larval rearing is considered a major bottleneck for the expansion of bivalve hatcheries required for the growth of the industry. Production of live microalgae is costly and labor intensive.
In addition, a diet of a single microalgae species is not optimal to support different developmental stages of bivalves, necessitating the cultivation of several microalgae species [3].
The efficiency of microalgae cultivation is determined by the amount of biomass produced and its biochemical composition. One of the ways to increase the productivity of microalgae cultivation is the use of phytohormones [4].
Auxins are key regulators of almost all plant growth and development processes, including flower organ formation, vascular tissue formation, shoot growth, phototropism, and gravitropism. However, the mechanisms determining these processes are still far from being fully understood. In general, it is believed that the formation of plant organs is determined by local auxin gradients, the creation of which involves PIN-family proteins that control the directions of auxin fluxes and the general distribution of auxin concentrations [5]. Several compounds found in plants and having auxin activity are known. These are primarily indole-3-acetic acid, with much smaller amounts of indole-3-butyric acid and phenylacetic acid, which have very weak activity. Indole-3-acetic acid is the major and universal auxin in all plants. Auxin synthesis occurs in almost all plant tissues, but its intensity varies greatly [6].
Rapid changes or fluctuations in auxin concentration at the level of an individual cell can initiate or terminate certain developmental processes. Auxins also mediate the transmission of interactions between cells, tissues, and organs over both short and very long distances [7, 8].
Auxin is an essential phytohormone that functions as a signaling molecule to stimulate both growth and developmental processes. The key role of auxin as an integrator of environmental signals has become evident in recent years, and emerging evidence suggests that auxin biosynthesis is an important component of general mechanisms of plant stress tolerance [9].
Bioassays, high-performance liquid chromatography, mass spectrometry, and some other physicochemical analyses, along with other indirect evidence, prove the existence of auxin or auxin-like compounds in many algal species [10, 11].
The presence and action of auxins have been shown in both unicellular and multicellular algae [12-14].
Knowledge of the mechanisms of action of auxins on unicellular algae opens the possibility of practical application of exogenous regulators of metabolism in increasing the bioproductivity of cell cultures.
The marine diatom Phaeodactylum tricornutum is known as a model object for studying the of physiology, biochemistry, and genomics of microalgae [15]. The ability of microalgae to accumulate a significant amount of lipids, up to 30% of dry weight, is known, which substantiates the potential use for biodiesel [16]. Due to its high growth rate, the ability to accumulate significant amounts of various metabolites such as proteins, carotenoids, carbohydrates, polyunsaturated fatty acids, this diatom is widely used as a live feed in aquaculture [17]. Previously, the stimulating role of salicylic and abscisic acids in the increase of biomass and fatty acids during the stationary growth phase was reported for Ph. tricornutum [18, 19].
An analysis of available literature data has shown a high potential for regulation of the biochemical composition of microalgae to be used in practical applications [20].
The objective of our study was to determine the optimum concentrations of plant hormones, indole-3-butyric acid (3-IBA) and indole-3-acetic acid (3-IAA), for the growth of Ph. tricornutum and their effect on biochemical parameters during the growth of microalgae.
Materials and methods
It our study, we used a culture of the diatom Phaeodactylum tricornutum from the collection of the Far Eastern State Technical Fisheries University (Dal’rybvtuz), Vladivostok. The algal culture was grown in the enrichment mode in an f/2 growth medium prepared on filtered and sterilized seawater and supplemented with solutions of basic mineral salts (NaNO3; NaH2PO4 ∙ H2O; Na2SiO3 ∙ 9H2O), trace elements (CuSO4 ∙ 5H2O; ZnSO4 ∙ 7H2O; CoCl2 ∙ 6H2O; MnCl2 ∙ 4H2O; Na2MoO4 ∙ 2H2O; EDTA-Na2; FeCl3 ∙ 6H2O), and vitamins (B1, B7, B12) [19]. The algae culture was maintained under constant conditions: temperature, 21-23 °C; illuminance, 8-10 klx; light: dark cycle, 8 : 16 h; and periodic stirring (4-5 times a day).
We used 3-IBA and 3-IAA as growth stimulants (Hebei Guanlang Biotechnology Co., Ltd, China).
Glass heat-resistant 1-liter conical flasks were used as cultivators. At the beginning of the experiment, we poured 400 ml of pure filtered and sterilized seawater, 100 ml of microalgae culture, and a stimulant at the studied concentrations into the flasks. One flask was a control, i. e. the culture grew without the growth stimulant added.
Microalgae were cultivated in a monoculture. Increase in algae biomass was detected as an increase in the number of cells counted in each experiment in three Goryaev’s chambers under a light microscope. The duration of the experiment was 14 days.
Total carbohydrate content was estimated by the method of acid hydrolysis of algae suspension samples, where formed monosaccharide units turned into furfural derivatives which, after supplementing the solution with L-tryptophan, formed colored complexes that absorbed light at a wavelength of 540 nm [21].
Sample preparation for protein measurements was carried out according to [22]. Protein content was measured by the Lowry’s method [23].
Total lipid content was measured by a method based on the color reaction of aniline with lipids in an acidic medium with intense color formation. Hydroxyl and carbonyl were the chromogenic groups [24, 25].
Total chlorophyll was extracted by acetone extraction from pre-frozen algae biomass [25]. Quantitative content of chlorophylls was measured spectrophotometrically at wavelengths of 630, 647, 664, and 750 nm. As a control, 90% acetone was used [26].
The results were processed using the Excel and STATISTICA® 7.0 software packages.
Results and discussion
We assessed the effects of various concentrations of 3-IBA and 3-IAA on the growth parameters of the Ph. tricornutum culture (Fig. 1, 2).
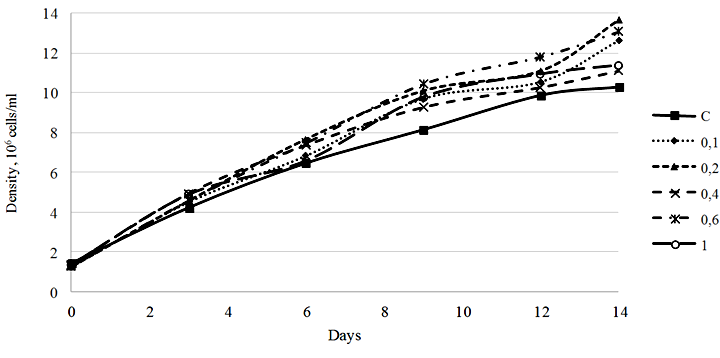
Fig. 1. Density dynamics of the Phaeodactylum tricornutum culture exposed to 3-IBA (10–5 М): C – control
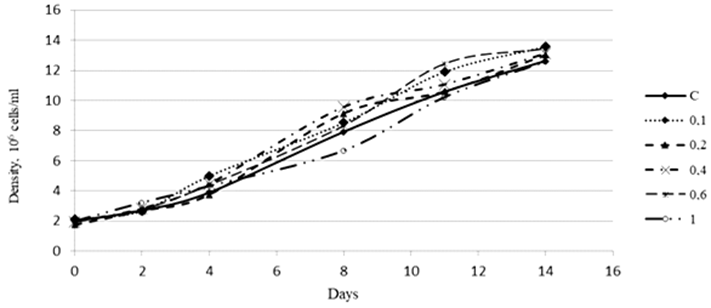
Fig. 2. Density dynamics of the Phaeodactylum tricornutum culture exposed to 3-IAA (10–5 М)
Our study showed that 3-IBA in a concentration range of 0.1-1.0 ∙ 10–5 М stimulated the growth of the microalgae culture. The most pronounced growth stimulation effect was observed in the culture exposed to 3-IBA at a concentration of 0.2 ∙ 10–5 М. The density of the culture on day 14 of cultivation amounted to 13.67 ∙ 106 cells/ml, with the initial density of 1.25 ∙ 106 cells/ml, which is equivalent to a 1 093.6% increase. It should be noted that the culture density increase in the control group for the same period was 720.3% (Fig. 1).
An assessment of the effect of 3-IAA on the growth of the Ph. tricornutum culture showed that high concentrations of this plant hormone in different periods of cultivation either inhibited the development of the culture or did not have a significant effect on its growth. A concentration of 3-IAA equal to 0.1 ∙ 10–5 М had an insignificant stimulating effect on the culture density (Fig. 2).
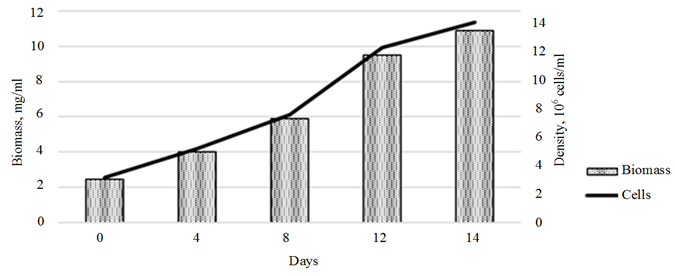
An assessment of the effect of the plant hormones on the growth of the control Ph. tricornutum culture showed a linear biomass growth within the first 10 days of cultivation, with a slowdown in growth rates by day 14 of cultivation. The overall rate of biomass growth over 14 days of cultivation amounted to 444.9% (10.9 mg/ml) (Fig. 3, a).
a
b
Fig. 3. Dynamics of the growth of the control Phaeodactylum tricornutum culture (a)
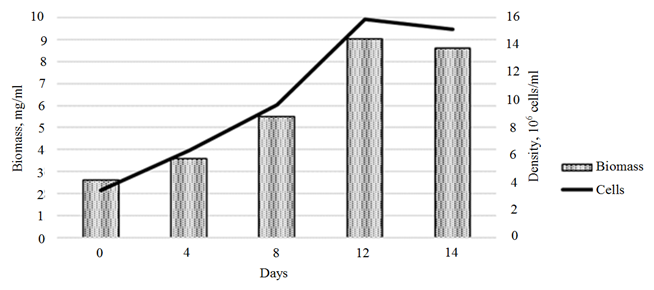
and the biomass of the cultures exposed to 3-IBA (b)
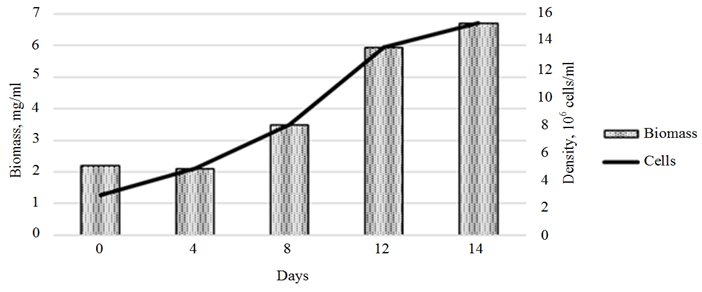
c
Ending of fig. 3. Dynamics of the growth of the Phaeodactylum tricornutum culture
and the biomass of the cultures exposed 3-IAA (c)
The stimulating effect of 3-IAA on the growth of Ph. tricornutum biomass was observed throughout the cultivation period (Fig. 3, с). The maximum growth rate, which amounted to 0.62 ∙ 106 cells/day, was recorded in the period from days 8 to 12 of cultivation. The total growth of microalgae biomass increased, compared to the control, by 328.2% (8.6 ∙ 106 cells/day).
The introduction of 3-IBA into the Ph. tricornutum culture medium provided a maximum growth of microalgae biomass on day 12 of cultivation (Fig. 3, b). The biomass growth rate for this period amounted to 303.2% (6.7 ∙ 106 cells/ml).
In our study, we assessed the effect of the plant hormones on the biochemical parameters of the Ph. tricornutum culture.
The dynamics of values of quantitative protein content in the control Ph. tricornutum culture showed a sharp increase (922%) in the protein concentration on day 4 of cultivation (Fig. 4), further cultivation did not have a significant effect on this parameter.
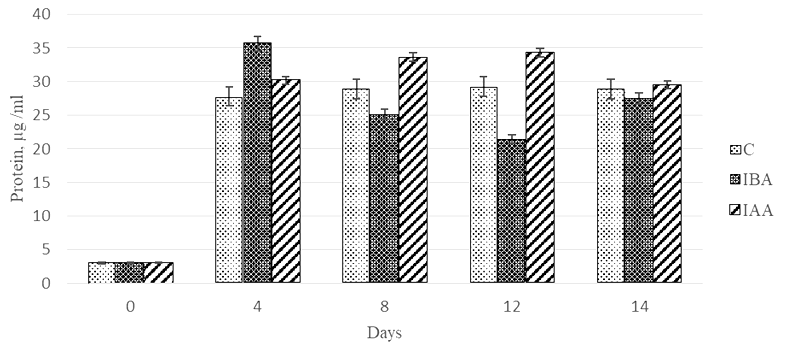
Fig. 4. Dynamics of values of quantitative protein content
in the Phaeodactylum tricornutum culture exposed to the plant hormones
The stimulating effect (1 179%) of 3-IBA on protein synthesis by the microalgae culture was observed on day 4 of cultivation. In this microalgae group, the protein concentration was by 27.9% higher compared to the control. The further cultivation of microalgae with 3-IBA led to a 60% decrease in the values of the parameter by day 12 of cultivation.
The stimulating effect of the plant hormone 3-IAA on the protein synthesis was recorded on days 8 and 12 of Ph. tricornutum cultivation. The protein concentration in these periods was 1.2-fold higher than in the control. A comparison of the effects of the two plant hormones showed that 3-IAA on days 8 and 12 of cultivation was 1.3-1.6-fold more effective as a protein accumulation stimulant than 3-IBA.
It should be noted that on day 14 of cultivation, the control culture and the experimental cultures did not differ in terms of protein concentration.
An assessment of the effect of the plant hormones on the stimulation of lipid accumulation showed that throughout the cultivation time 3-IBA did not have any effect on this parameter compared to the control (Fig. 5).
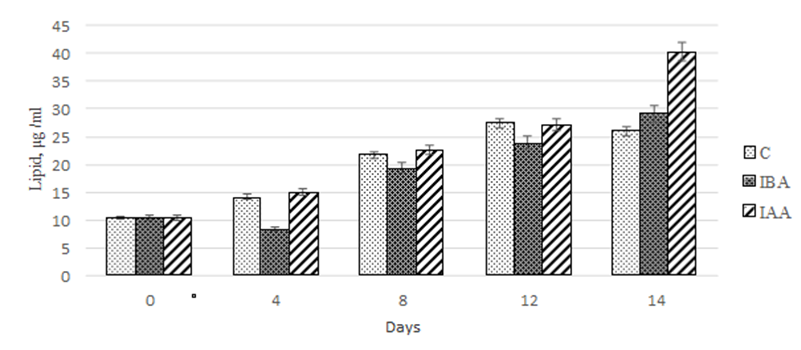
Fig. 5. Dynamics of values of quantitative lipid content
in the Phaeodactylum tricornutum culture exposed to the plant hormones
In both groups, the maximum accumulation of lipids was observed on days 12-14 of cultivation: 260-280% relative to the data at the beginning of the experiment.
Within the first 12 days of cultivation, both plant hormones under study equally stimulated the lipid accumulation in the Ph. tricornutum culture. However, on day 14 of cultivation with the exposure to 3-IAA, it amounted to 40.3 µg/mL, which was 1.5-fold greater than in the control and in the group exposed to 3-IBA.
When assessing the quantitative content of carbohydrates, we observed a nonlinear increase in values of this parameter on day 14 of cultivation (Fig. 6).
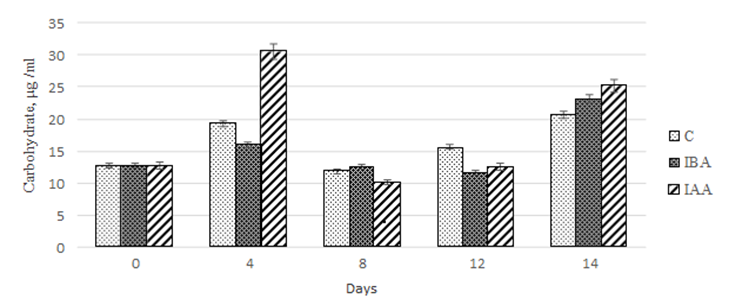
Fig. 6. Dynamics of values of the quantitative content of carbohydrates
in the Phaeodactylum tricornutum culture exposed to the plant hormones
The maximum carbohydrate accumulation in the control culture was recorded on days 4 and 14 of cultivation. However, on days 8 and 12, there was a 1.6- and 1.2-fold decrease, respectively, compared to day 4 of cultivation.
During our study, we found that 3-IBA did not have any effect on the carbohydrate accumulation in the Ph. Tricornutum culture within the first 12 days of cultivation.
However, on day 14 of cultivation, the increase in carbohydrate concentration was 82.2% relative to the initial value in the starter culture. However, the values obtained did not differ from those for the control group.
The effect of 3-IAA on the carbohydrate accumulation in the microalgae culture was similar to that described for 3-IBA. On day 4 of cultivation, the concentration of carbohydrates in the culture increased by 240% compared to the control. On days 8 and 12 of cultivation, there was a 3.0-2.4 decrease in the carbohydrate concentration, respectively, compared to the values on day 4 of cultivation. At the end of the experiment (14 days), we did not find significant differences in the carbohydrate concentration between the experimental cultures (Fig. 6).
Synthetic and metabolic processes in microalgae are determined by the efficiency of photosynthetic processes, including the chlorophyll concentration.
As our study showed, the chlorophyll concentration in the experimental and control cultures increased within the first 8 days of Ph. tricornutum cultivation. For the control culture and the culture exposed to 3-IBA, the increase in chlorophyll concentration was 842.9 and 927.4%, respectively (Fig. 7).
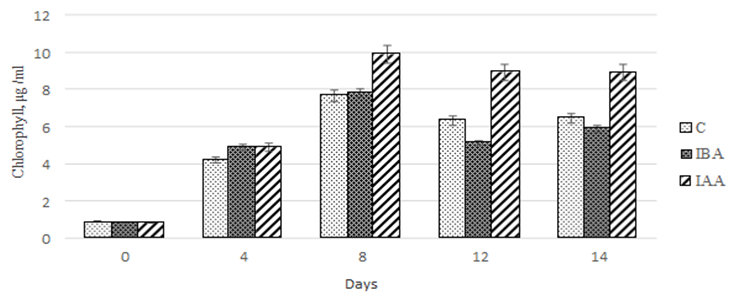
Fig. 7. Dynamics of values of the quantitative chlorophyll content
in the Phaeodactylum tricornutum culture exposed to the plant hormones
In the microalgae culture exposed to 3-IAA, the increase in chlorophyll concentration proved to be more significant and amounted to 1 178.6%. The further cultivation to 14 days did not cause significant changes in the chlorophyll concentration in the culture exposed to 3-IBA.
However, in the control culture and the culture exposed to 3-IBA, the chlorophyll concentration decreased on days 12 and 14 of cultivation.
Conclusion
In this study, we assessed the effects of auxin plant hormones (indole-3-butyric acid and indole-3-acetic acid) at various concentrations on the microalga Phaeodactylum tricornutum.
The study of the effect of auxin hormones on the growth of Ph. tricornutum showed that 3-IBA at a concentration of 0.2 ∙ 10–5 M had the greatest stimulating effect on the microalgae growth at 14 days of the experiment. We found that 3-IAA at high concentrations exerted an inhibitory effect on the growth of the culture. The stimulating effect on the microalgae growth was recorded for 3-IAA at a concentration of 0.1 ∙ 10–5 M. Also, we observed a stimulating effect of 3-IAA at this concentration on the growth of Ph. tricornutum during 14 days of cultivation.
An assessment of the effect of the auxins under study on the biochemical composition of Ph. tricornutum showed a stimulating effect of 3-IAA on the protein increase on day 12 of cultivation. The protein concentration was 1.2-fold higher compared to the control group. We found that 3-IAA exerted the most pronounced stimulating effect on the accumulation of lipids in the culture. The lipid content in this group on day 14 of the experiment was 1.5-fold higher than in the control. Also, 3-IAA had the maximum effect on the carbohydrate content of the culture. The maximum value of this parameter, recorded on day 4 of the experiment, was 1.5-fold higher than the carbohydrate content in the control group. The positive effect of 3-IAA on the dynamics of chlorophyll accumulation in the medium compared to 3-IAA and the control group deserves special mentioning. The maximum for this parameter in the case of 3-IAA exposure was recorded on day 8 of the experiment.
As a result of our study, we found that 3-IAA has a positive effect on the growth of Ph. tricornutum. We observed a multidirectional action of both 3-IBA and 3-IAA at optimum concentrations on the biochemical parameters of Ph. tricornutum.
Thus, the study has made it possible to identify the effects of the plant hormones of different chemical natures on the growth characteristics of Ph. tricornutum. We have determined the effective concentrations of the plant hormones and their effects on the culture growth and on the biochemical composition of the cultures of the microalgae species analyzed.
1. The State of World Fisheries and Aquaculture. Meeting the sustainable development goals. Rome: FAO, 2018. 227 p. URL: https://www.fao.org/3/I9540EN/i9540en.pdf (data obrascheniya: 13.04.2023).
2. Helm M. M., Bourne N., Lovatelli A. Hatchery cul-ture of bivalves: a practical manual. FAO Fisheries Tech-nical Paper 471. Food and Agriculture Organization of the in the laboratory // Marine Biology. 2004. V. 15. P. 350-355.
3. Utting S. D., Spencer B. E. The Hatchery Culture of Bivalve Mollusc larvae and juveniles // Ministry of Agriculture, Fisheries and Food, Directorate of Fisheries Research Laboratory leaflet number. 1991. V. 68. P. 32.
4. Kovalev N. N., Leskova S. E., Mikheev E. V. Hetoceros: modulation of growth and composition under the action of salicylic acid // Agrarnaya Rossia. 2022. N. 10. P. 13-18.
5. Ljung K., Bhalerao R. P., Sandberg G. Sites and ho-meostatic control of auxin biosynthesis in Arabidopsis during vegetative growth // Plant Journal. 2001. V. 28. P. 465-474. DOI:https://doi.org/10.1046/j.1365-313x.2001.01173.x.
6. Ludwig-Müller J., Bendel U., Thermann P., Ruppel M., Epstein E., Hilgenberg W. Concentrations of indole-3-acetic acid in plants of tolerant and susceptible varieties of Chinese cabbage infected with Plasmodiophora brassicae // New Phytologist. 1993. V. 125. P. 763-769. DOI:https://doi.org/10.1111/j.1469-8137.1993.tb03926.x.
7. Rozov S. M., Zagorodskaya A. A., Deineko E. V. Auxin: biosynthesis, metabolism, transport // Uspehi sov-remennoy biologii. 2013. V. 133. N. 1. P. 50-62.
8. Normanly J., Slovin J. P., Cohen J. D. Auxin biosynthesis and metabolism // Plant Hormones. Biosynthesis, signal transduction, action. 2010. P. 36-62. DOI:https://doi.org/10.1007/978-1-4020-2686-7_3.
9. Mroue S., Simeunovic A., Robert H. S. Auxin production as an integrator of environmental cues for developmental growth regulation // Journal of Experimental Botany. 2018. V. 69 (2). P. 201-212. DOI:https://doi.org/10.1093/jxb/erx259.
10. Cooke T. J., Poli D. B., Sztein A. E., Cohen J. D. Evolutionary patterns in auxin action // Plant Molecular Biology. 2002. V. 49. P. 319-338.
11. Tarakhovskaya E. R., Maslov Y. I., Shishova M. F. Phytohormones in algae // Russian Journal of Plant Physiology. 2007. V. 54. P. 163-170.
12. Garcia-Jimenez P., Rodrigo M., Robaina R. R. Influence of plant growth regulators, Polyamines and Glycerol interaction on growth and morphogenesis of carposporelings of Grateloupia cultured in vitro // Journal Apply Phycology. 1998. V. 10. P. 95-100.
13. Bradley P. M., Cheney D. P. Some effects of plant growth regulators on tissue cultures of the marine red alga Agardhiella subulata (Gigartinales, Rhodophyta) // Hydrobiologia. 1990. V. 204/205. P. 353-360.
14. Basu S., Haiguo S., Brian L., Quatrano R. L., Muday G. K. Early embryo development in Fucus dis-tichus is auxin sensitive // Plant Physiology. 2002. V. 130. P. 292-302. DOI:https://doi.org/10.1104/pp.004747.
15. Smet I. D., Vob U., Lau S., Wilson M., Shao N., Timme R. E., Swarup R., Kerr I., Hodgman C., Bock R., Bennett M., Jurgens G., Beeckman T. Unraveling the evolution of auxin signaling // Plant Physiology. 2011. V. 155. P. 209-221.
16. Haro P., Sáez K., Gómez P. I. Physiological plasticity of a Chilean strain of the diatom: The effect of culture conditions on the quantity and quality of lipid pro-duction // Journal of Applied Phycology. 2017. V. 29. N. 6. P. 2771-2782.
17. Branco-Vieira M., Martin S. S., Agurto C., dos San-tos M. A., Freitas M. A. V., Mata T. M., Martins A. A., Caetano N. S. Potential of Phaeodactylum tricornutum for Biodiesel Production under Natural Conditions in Chile // Energies. 2018. V. 11. P. 54-69. DOI:https://doi.org/10.3390/en11010054.
18. Xu J., Fan X., Li X., Liu G., Zhenyan Zhang Z., Zhu Y., Fu Z., Qian H. The effect of salicylic acid on the accumulation of fatty acids in Phaeodactylum tricornutum in the stationary growth phase // Journal Applied Phycol-ogy. 2017. V. 29. P. 2801-2810. DOI:https://doi.org/10.1007/s10811-017-1191-6.
19. Zhang H., Yin W., Ma D., Liu X., Xu K., Liu J. Phytohormone supplementation significantly increases fatty acid content of Phaeodactylum tricornutum in two-phase culture // Journal of Applied Phycology. 2021. V. 33 P. 13-23.
20. Guillard R. R. L. Culture of Phytoplankton for Feeding Marine Invertebrates // Culture of Marine Inver-tebrates Animals. New York: Plenum Press, 1975. P. 29-60. DOI:https://doi.org/10.1007/978-1-4615-8714-9_3.
21. Laurens L. M. L., Dempster T. A., Jones H. D. T., Wolfrum E. J., Wychen S. V., McAllister J. S. P., Rencen-berger M., Parchert K. J., Gloe L. M. Algal biomass constituent analysis: method uncertainties and investiga-tion of the underlying measuring chemistries // Journal of Analytical Chemistry. 2012. V. 84. N. 4. P. 1879-1887.
22. Herbert D., Phipps P. J., Strange R. E. Chemical analysis of microbial cells // Methods in Microbiology. 1971. N. 5. P. 209-344.
23. Lowry O., Rosenbrougt N., Parr A., Randall R. Protein measurement with the Folin phenol reagent // Journal of Biological Chemistry. 1951. V. 193. N. 1. P. 265-276.
24. Johnson K. R., Ellis G., Toothill C. The sulfophos-phovanilin reaction for serum lipids: a reappraisal // Clinical Chemistry. 1977. V. 23. P. 1669-1673.
25. Sarneiro M., Pojo V., Malcata F. X., Otero A. Lipid accumulation in selected Tetraselmis strains // Journal of Applied Phycology. 2019. V. 31. N. 5. P. 2845-2853.
26. Aminot A., Ray F. Standard procedure for the determination of chlorophyll a by spectroscopic methods. ICES techniques in marine environmental sciences // International Council for the Exploration of the Sea. 2001. 16 p.
















